Hot damn, ah gots mah vacuum chamber and bodaceously big pump, so where do ah go fum heah?
Goooood question, so lets hit on a few key points. There is more to vacuum purging and finishing than just sticking the oil in the chamber, and turning on the pump.
Tip # 1 Selecting vacuum levels and pumps. Don't waste money and cannabinoids purchasing a 2 stage vacuum pump.
First lets look at what is going on when you place oil in a vacuum chamber and start raising the temperature.
The boiling points of compounds are given at a specific elevation, normally sea level. That is because the weight of the atmosphere at sea level is about 14.7 psi, 760, 000 microns or millibars, or 29.92" Hg. The weight of that atmosphere suppresses the boiling point, and if you remove that weight, the compounds boil at lower temperatures.
That is a good thing in that it allows us to cold boil away solvents with little heat, but it has its limitations, and one is that the same vacuum also changes the boiling point of the components suspended in the alcohol, that you are hoping to end up with when the alcohol is gone.
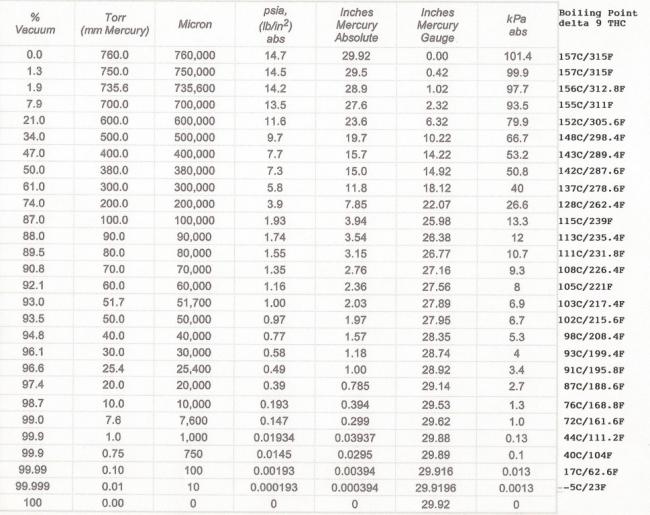
For instance, here is a chart, complements of Skyhighler, demonstrating what happens when you pull a vacuum on THC. Note that at sea level, Delta 9 THC boils around 157C/315F.
Now look at what happens at -29.9196" Hg, where Delta 9 THC is boiling at only minus -5C or 23F.
Whoaaaaa! That sort of flies in the face of the common perception that nothing but a 2 stage vacuum pump will suffice, when most single stages will pull to below 100 microns, which is about -29.916" Hg.
Tip # 2 Install a ball valve between the vacuum chamber and the vacuum pump, so when the pump is turned off, the vacuum line can be closed off, so as to not suck vacuum oil from the pump crank case, into the vacuum chamber.
Better yet, install a cross, with the pump ball valve in the straight through port and a vacuum gauge in one of the side ports, leaving the last port for another ball valve, which will allow backfilling directly from the atmosphere.
We install a filter on this port, so as to not vent dust into a fresh extraction.
Tip#3 The brains of the system is the compound pressure gauge, so get a quality unit and calibrate it at the start of the process, as well as periodically check it to see that it is returning to zero.
Bourdon gauges have flat partial circle shaped thin walled tube inside, that opens and closes its diameter under vacuum, and they take a little time to nestle in when new.
We like a Just Better M2-250B Refrigerant Compound Gauge ourselves, which has removable front cover, and a calibration screw that allows zero adjustment.
Never try to turn the adjustment screw under pressure, or you will most likely damage the adjustment screw.
Tip#4 Check your pump oil for color, clarity, and level before starting the process and regularly during use, most especially during cold boiling under vacuum.
We prefer a clear, colorless pump oil, so we can see if it is contaminated by looking at it through the oil level view window. You can also note the level, as most have an upper and lower limit indicated.
All slide vane vacuum pumps puke oil and oil vapors out the exhaust and are noisy, so it will make a mess where you set it, unless you add a larger filter on the exhaust.
I just saw a cute conversion, that allowed spinning a Fomoco size oil filter on the exhaust, to contain the mess. If you don't have such an arrangement, might I suggest you use a metal tray under your pump where making a mess is of concern.
If you are using the pump to cold boil, and aren't using an effective cold trap, you must regularly change your oil as the level rises beyond the safe level and begins to puke out the exhaust.
More on cold traps further down.
Tip#5 Test your vacuum pump by itself, before trying to vacuum test the complete system.
You can test your vacuum pump to see what level it will achieve in heaven, but simply attaching a refrigerant hose to the pump and the one to a vacuum gauge.
Make sure that the cord connecting the pump is heavy enough for the pumps rated amperage and avoid using extension cords.
Don't run multiple motors on the same circuit, because of the high locked rotor amp spike during startup.
Turn on the pump and see how far it pulls down. If it doesn't pull down to its rated capacity at your altitude, check for leaks at each of the hose connection points.
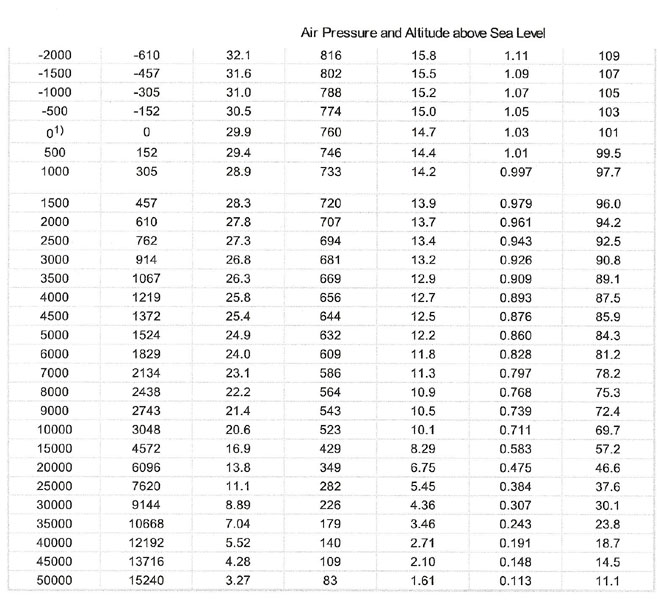
To adjust for altitude, here is a good chart, taken from: http://www.engineeringtoolbox.com/air-altitude-pressure-d_462.html
Tip#6 Vacuum test your complete system by first pumping it down as far as it will pull down, and turning off the pump isolation valve. There should be no pressure loss after at least an hour.
Except for losses due to temperature changes, there shouldn't be any pressure losses overnight. The key point is that the system needs to be hermetically sealed.
Tip#7 To keep the pressure of your chamber within the range that you have selected for optimum results, open the bleed valve and spill air into the pump line.
If you have both a 1/4" and a 1/2" hose connection on your pump, you can install a bleed valve on the unused one, to throttle vacuum at the pump.
Besides acting to limit how low your pump pulls the negative pressure down to, injecting dry air at that point, will help dry out the crank case vapors and slow down crankcase dilution.
Tip#8 Regularly close the ball valve to isolate the vacuum pump, and turn it off to give it a rest.
It isn't necessary to continue to run the pump, once the desired pressure is reached, but it is necessary to regularly start the pump and open the valve to burp it, so as to keep the atmosphere enriched by the cold boiling solvent removed.
You can also valve off the chamber and introduce a dry gas through the other vacuum pump port, to act as a ballast to dry out the crankcase.
Clearly you can't pull much of a vacuum venting a dry gas into the intake port, but the valved off chamber vacuum loss is tolerable for a few minutes.
Tip#9 Use a cold trap between the vacuum chamber and the pump when cold boiling solvent to recover the solvent for reuse, before it dilutes the vacuum pump oil.
To insure that the solvent is recovered in the cold trap, and not cold boiled away there too, you must control the vacuum pressure at the cold trap, as opposed to the chamber.
The lower the cold trap temperature, the more efficient. Besides ice water, antifreeze, dry ice and alcohol, and liquid nitrogen are used in cold traps to achieve desired recovery rates.
A trap with more face area to the flow, will decrease velocity and increase transfer time.
A trap with the most surface area will transfer the most heat, at the same velocity and temperature.
Tip # 10 When you first pull a vacuum on a liquid, it will outgas the dissolved gasses and may boil explosively and violently, until the gasses are expelled.
To prevent making a mess, throttle the vacuum carefully, while observing the boiling action, until the gasses are expelled and the pool calms down.
Tip # 11 When cold boiling, use a glass sheet or equivalent cover over your boiling pan, so as to not blow specks of oil all over the interior surfaces of your chamber or oven.
Even though it may look like boiling has stopped, it is still sublimating, with occasional explosive bumping.
Tip # 12 Before paying good money for a cheaply made vacuum chamber, consider making something of your own like the one shown at:
TIP #13 How come I had violent boiling when I first pulled a vacuum, and then the boiling slowed down to a crawl?
The liquid in the chamber has dissolved gases from the atmosphere in it and when you lower absolute pressure, they boil out and escape.
If it is boiling too violently to contain, try throttling the vacuum pressure back some, until most of it escapes, and it calms down.
TIP#14 How to calibrate a vacuum gauge without a reference standard
Here is another one from Skyhighler, on how to calibrate a vacuum gauge, accurately enough for our purposes, when you don't have a calibrated reference standard to compare it to.
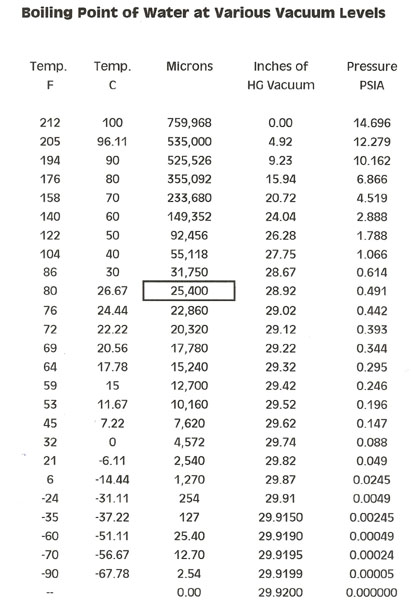
Here is a chart showing at what temperature and pressure distilled water boils and some ideas on how to use it.
If you track the temperature of a pool of water, when you pull a vacuum on it, several things happen.
As the partial pressure is pumped closer to absolute zero, the dissolved gasses boil out of the pool.
The water itself starts to boil.
The temperature of the pool of water drops due to the refrigerative effects of the water boiling under vacuum.
The boiling slows down because the pools temperature drops closer to its boiling point.
Sooooooooooo, if you were to take distilled water and degas it by pulling a hard vacuum on it until violent boiling ceases, it can be used as a calibration standard that is close enough to track our process.
As it predictably boils at different temperatures at a specific absolute pressures, you can measure the temperature that the water is boiling at and find the corresponding absolute pressure in the following chart.
If you leave the thermometer or probe in the puddle and pull a vacuum on the degassed water, it will come to a boil at some vacuum level. Look that temperature up on the chart, and it will tell you at what vacuum level you are at.
For instance, at -29.5" Hg vacuum, the water should boil at around 11C/52F after degassing.





Bingo!
Bingo!
Whatsapp: +195.464.18238 Open link https://sites.google.com/view/nembutal-pentobarbital-sodiums Buy Xanax bars , Lean syrup , Oxycodone , Adderall , Percocet , Methadone , Ecstasy , Lsd and acid , Actavis Promethazine Cough Syrup , Viagra , Vyvanse , Klonopin , Fentanyl , Rohypnol (Roofies) , Ketamine , Hydrocodone 10/325, MDMA (molly) crystal and pill form , Crack Cocaine , Heroin (white, brown and tar) Weed , Marijuana , Cannabis buy in Switzerland , Germany , Spain , Italy , Strawberry , Sour Diesel , Jack Herer , Durban Poison , Haze, Pineapple Express , Blue Dream , Purple Haze , AK-47 , Grapefruit , White widow , OG Kush , Purple Kush , Black weed , CBD Oil , Hemp oil , Moonrock for sale in Switzerland , Germany , Spain , Italy ,E-mail: danny@ doctor. com https://onlinecannamedshop.com
I'm confused though at 115 wouldn't you end up decarboxylating THC? According to my calculations thc de carbs at 109.4 at 29.5 mmhg. Or did I miss calculate?
What would you recommend my temp and inHg pressure be for making shatter at 5,300 feet elevation? Full vacuum at 5,300 is -26 Temp is ?
At 5300 feet, there is only about 25.17" Hg to start with, so you have an adjustment factor of about 4.75", so would suggest -24.75" Hg at 115F. GW
Hi guys, I have a big problem with my oils. Not a lot of problems making shatter and I love it. I follow gray wolf method (thin film vaccum 115f @ 29.5 hg) and sometimes I have hard shatter. My problems are when I want to do some wax honeycomb. All people say I have to go to 135-140 f. But a that temp my oils became always liquid and it seem normal to me. What am I wrong? Here in this fucking Spain people ask for wax or honeycomb so I have to learn that. The only time I made wax -honeycomb is when I put temp at 90-95 for many hours. Why? How I can solidify my bho in honeycomb consistency? How I can have a solid products? Maybe the liquid shit I see needs to be dry at room temp? I'm in tilt and I' m close to became crazy with those oil.
Depends on your starting material because different strains have different amounts of inactive ingredients, more inactive ingredients means less pure results wax or honeycomb. Shatter and winterized concentrates have less inactive ingredients because of purging processes.
I am asking me if we not already start a decarbolisation with the purging as we set the boiling point down?
Easy for you to say! Might you please elaborate on your question? GW
I interpret your question as, "If you do not wish to decarboxylate the material during purge, do you use lower temperatures?" If that is your question, the answer is yes. To vacuum purge, the viscosity of the material needs to be low enough for the bubbles to readily escape. Heat lowers the viscosity, so I heat the material in a thin film until it reaches that state, but no hotter. Usually between 100F/115F. I use 125F for ethanol purging, because otherwise the solution blows enormous bubbles that defy bursting without bumping vacuum levels. GW
What about the Freezing Point of Alcohol/Ethanol Under Vacuum? I haven't found that one yet?
I am in anchorage alaska. Can anyone please help me understand the elevation conversion chart for my elevation. Google tells me its 102.
You have to extrapolate. Sea level is 29.9" Hg and 500 feet elevation is 29.4" Hg. 29.9 - 29.4 = .5 .5/500 X 102= .102" Hg pressure loss at 102 feet elevation, which leaves 29.798" Hg. Roughly one inch per 1000 feet pressure drop. GW
Just get stronger better pumps everyone and you wont have to worry about all this elevation stuff so much. Unless you live in the himalayas or in a crator miles below sea leval
Sorry if i cone across rude, i font mean too. But in the beginning of this article you warn us about delta9 reaching boiling point with pumps that are to strong. So what is the lowest cfm/hp one should aim for? Is there a way to calculate it without actually buying and testing the vacuum pump? Im eager to learn all I can about this!
What turns BHO black? To much heat? I live in Oregon, happy smoking. I keep getting amber oil after the initial run but after I purge it, it turns dark. Is the heat to high? I check the heat with an IR gun and it's always the exact same, 105°F. Should I raise the temp? Wax taste great after a two day purge, no butane left that I can tell.
The C-30 anthro cyanin plant pigments add the most color, and exhibit the Beers/Lambert effect. A thin layer may be bright and clear, while two thin layers may approach opacity. They are also glucosides (plant sugars) which caramelize and darken with heat. We extract at subzero temperatures to minimize our C-30 molecule pickup. GW
What is the best way to seal a rip in the silicone gasket for the vacuum chamber?
I would suggest replacing the gasket. GW
You can flip the gasket over for many many vacs, if it looks like u can, what will hurt to try?
It's science but not complicated if you know what your desired output should be. Took about two weeks of experimentation but I've achieved my desired output and have done so every time now without fail. Good luck to you'all and happy dabbing!!! I've got something new I've been working on but it's very complicated. I'm talking A-THC. : )
so i have only been closed loop extracting for a short period of time n i have the extraction end prosses down decent on color n pull. my only question is on the vac purg side ive been trying to make crumble/cookie and have been getting more of a unstable shatter look id like to know what people are using fir temps and vac pressure and how long they are letting it sit in oven i need a step by strep proses to achive a stable crumble ?? plz help
anyone know if Shatter will re set if I was to reheat and let set? I ask because I've got several small runs and would like to make it one large slab :D
Heat it in a bowl set in a crockpot on low, it will only take a couple min, use a temp gun and do not let it go over 90%
Any one want to know how real chemistry guy would do it and what there not telling you here or anywhere? I use a 12 liter round bottom reaction vessel with 3 neck head heated by a glas col mantle a liebig condenser and receiving flask. Vacucum is a diaphragm vacuum pump which can pull about -740mmhg. If you are using an oil sealed vac you got from harbor freight, your stupid.your making poison. I will also note that I have a thermometer and manometer on my apparatus. You should always know the temp and pressure of your system. If you don't, stop your stupid.stop killing people slog. Because your vac gauge on your $150 pump says you are at 29mmhg does not make it so. The science of pulling a full vacuume on planet earth is a very deep and complex. And it is in fact impossible. At best anyone here is doing an intermittent distillation under reduced pressure. Not a vacuum. Sorry harbor freght pumps and rice cookers. A real lab pump will cost you at least $500 for a weak one that can maybe pull 22-23 hg. Mine is about $1000. Have control over the heat. Hot plate in a sealed home made chamber. Bad idea. Stop. At different temps and pressures different things are happening. Just microwaving a pyrex dish and throwing it in the chamber for a minute makes poison. Not thc. Your evap or purge taking several days? Your product is loaded with residual solvent I promise you.even when you think it's done, it's still there. In a nut shell, this is serious organic chemistry You people are attempting to Execute with crude equipment and zero knowledge. Research, invest in the propper equipment.
I've pulled a hundred microns with many different setups. A hundred microns wasnt good enough for what I was doing. You can get a hundred microns with some pretty outdated vane style pumps. And you (could) pick them up for not much back in the day. Just did a quick ebay search and you can find a used alcatel direct drive for 150 bucks. It'll get you to 100 microns easily. If you wanted to get anal about it, you could get a higher end pump and run a turbo vac inline with your mechanical pump and then youd get as close to a perfect vacuum as your going to get for under 10000. You could install some filters and traps which would help reclame solvents, protect your pump lubricant and ensure that no vapours got past your pumps exhaust filter.... Not really sure what the point of the post is. Beating your chest with your "knowledge" or??? It's not really rocket science.
It's not rocket science so why take risks? I checked everything he said and it makes sens. If you are going to concentrate pounds of weed in oz of thc spending 2000$ to do it right should not be an issue, especially if it guaranty you quality.
Crockpot on low filled with water, "forgot that" float te bowl in it with your stuff in a pile. Don't take long at all man
It seems that some loser is voting thumbs down for no reason on people's comments, get a life loser!
Are there any negatives to reclaiming isopropyl alcohol? I was considering buying a 3 gallon stovetop still so I can get my alcohol back for reuse
I use a Black and Decker rice cooker with a 3/8th vent hole in the glass lid, which it comes with.and attach a length of surgical tubing from the vent hole to a coil of 1/4" copper tubing which is essentially a still. You cool the coil with ice and collect the IPA into a container below. I can reclaim up to 80% of my alcohol... maybe more at times.
[…] Vacuum Purging and Processing Tips | Skunk Pharm … – Hi ive been reading alot on this site and love it but SWIM has been making bho and it seems to take a week to two weeks to vacuum purge this is bring the vac to full … […]
Is SWiM using heat? Vac pressures? Kinda like fine wine making maybe... you have to finesse the art of it If SWiM gets the great results...
Love this!
Hi ive been reading alot on this site and love it but SWIM has been making bho and it seems to take a week to two weeks to vacuum purge this is bring the vac to full vac leaving pressure sealed at -29.5 and turning the vacuum back on for a half hour every 4 hours or so to help pop bubbles. SWIM leave the patty on one side till no more bubbles then flips purges till no bubbles wondering if he is doin sumthin wrong or if he is one of the few doing it right because ive yet to hear of anyone purging this long but his oil is always the smoothest thanks love skunkpharm
Try thinning it out more to expose more surface area and lessen the thickness the butane needs to travel through to be pulled from the bottom of your patty to the surface. Also, introduce "atmosphere". Take it out for a few minutes, collect it, carefully spread and put back in oven.
He is not really pulling 29.5 nobody is unless your in a real lab. Then maybe. Know your temp and pressure and where your solvent should boil at those ranges
Hello GW. I would like to say congrats on the work you guys are doing. The info you guys are getting out is priceless and doing nothing but good for the cause. My question is about vacuuming oil. I don't understand why people think one MUST use a vacuum. It is my understanding that putting a material under vacuum lowers its boiling point. Therefore, there should be no difference, from a chemistry standpoint, if one was to take the oil up to 212F with water or 240F with cooking oil in a double boiler. Am I missing something? Could you respond to my email? I'm sure more questions will follow. Thank you and keep up the amazing work.
Vapor Pressure.
Correct vacuum is not necessary. One can distill the solvent at normal pressure and higher temps. We use vacuume to reduce these temps and protect volatile compounds
But those volatile compounds will evaporate more easily vacuum. Is it really protecting them?
This is a good point, and something I have been wondering myself. If the boiling point of the compounds dissolved in your solvent lower at the same rate as the solvent's boiling point itself then why use a vacuum? Forget about reclaiming solvent I'm talking purely from the perspective of removing solvent from desirables while losing the least amount of volatiles possible (terps). Presumably the boiling points of the terpenes and Cannabinoids lower at the same rate as the solvent's boiling point, so therefore pulling a vacuum would then make no difference in preserving those volatile compounds which we are attempting to isolate. Theoretically just being precise with heat and time would produce the desired result. As long as the boiling point of the solvent is lower than that of the solute why would lowering the atmospheric pressure help? Just heat the mixture to at or above the solvent boiling point, pulling a vacuum (in the situation where the solvent and solute boiling points lower at the same rate under the same vacuum) would not change the relationship of the solvent boiling point to the solute boiling point. So if terpenes, which is really the crucial element here in terms of being precise, start to boil off at 20 deg Celsius, lets say (that number is from Ed Rosenthal book Beyond Buds), then pulling any vacuum on your solution would immediately start boiling off terps, assuming your working at room temperature. It would seem from this perspective that not pulling a vacuum at all, or working under cool temperatures, would help to preserve those terps, since the solvent in question - in this case butane - will boil off at -1 deg Celsius. See, http://hemphacker.com/what-every-hash-oil-extraction-artist-needs-to-know-about-temperature/ for a little more on what the previous poster and I are referring to. Is this not true? (yes I could check the chart and compare percentage drops in boiling point vs pressures, but would love an actual explanation here) Do the boiling points of terpenes and cannabinoids move down at a different rate than the boiling point of the solvent? Im thinking they don't, but If someone would actually do justice to this question that would be nice, thanks.
Honestly, it comes down to a safety issue. Arguably, one is safer extracting flammable solvents at lower temps; vacuum extraction provides this (relative) safety.
It's not a liquid
Garbage info. All liquids evaporate when you remove all atmospheric pressure. Good thing you can't vac out gravity.....
[…] Vacuum Purging and Processing Tips | Skunk Pharm … – I’m lives at 140 ft above sea level and am going to be doing my first run. I am going to be vac purging in a 5 gal vac chamber and 3 cfm 2 stage pump under a hot … […]
I'm lives at 140 ft above sea level and am going to be doing my first run. I am going to be vac purging in a 5 gal vac chamber and 3 cfm 2 stage pump under a hot plate, my only confusion comes with the pressure I should be purging my oils at. If I run full vac -30hg would all my thc9 and terpens boil out? What pressure should I use and should I leave the pump running the entire time because I believe the unit was made to process oil.
Damm I neec to make some videos and help these people outt watching video of it done online is best and rember CRAP IN CRAP OUT if ur usen shit you will get shit!
[…] to someone that has predispositions against butain as a solvent for extracting cannabinoids.rnrnVacuum Purging and Processing Tips | Skunk Pharm Research LLCrnrnVacuum Equipment and Process Tips | Skunk Pharm Research LLCrnrnVacuum - Wikipedia, the […]
[…] to someone that has predispositions against butain as a solvent for extracting cannabinoids.rnrnVacuum Purging and Processing Tips | Skunk Pharm Research LLCrnrnVacuum Equipment and Process Tips | Skunk Pharm Research LLCrnrnVacuum - Wikipedia, the […]
Just curious why you guys use "hg measurements rather than mm hg, microns or torr(millitorr) . I find the "hg confusing. What type of gauge are you using that reads "hg? Thx. I am totally new at this, have not tried to do anything yet, am researching before I do anything, but am not new to high vacuum applications.
and.... what is the level of vacuum needed and how fast do you need to get there? could you talk in microns for me? Is backstreaming really an issue?? I mean we are talking not a lot of molecules here....that could be migrating back and forth. As far as pump ratings.... you guys are aware that these are rated in a perfect world at the outlet...right? ....and never in the amount of time that you would actually want to pull that level. What about diffusion pumps or turbo pumps?? you guys use these?? using stainless manifolds or glass and what type of gauges. Ever consider a rough pump first to pull the initial vapors out and then change over to a higher end finishing pump?? Thanks for answers. :) cheers
Ur dumb
I would like to build one of my own could you send me a parts list and were to get them by chance.
[…] Vacuum purging and processing tips | skunk pharm research llc […]
[…] Vacuum purging and processing tips | skunk pharm research llc […]
[…] Vacuum purging and processing tips | skunk pharm research llc […]
[…] Vacuum purging and processing tips | skunk pharm research llc […]
GAST DAA V155 oil less diaphram vacuum compressor. these are rated between 3.2-3.8 cfm and draw down to 25 microns, thinking it would work well along side my 3 gallon chamber any thoughts ? these are apparently used in medical and lab settings. can anyone shed some light on how a diaphram compressors differs from rotary vane ? pros and cons. I currently use a CPS 3 cfm single stage tech-set which works wonders but looking to upgrade to an oil less pump as ive heard they are the way to go
We've had good luck with a DAA 501 GB in some applications. They will pump some vapors, but are not chemically rated like a Welsh A rotary vane pump rotates a rotor with spring loaded vanes attached to the outside, in an irregular shaped cylinder, so that the spaces are alternately larger to intake and made smaller as it rotates, to discharge. A diaphragm pump works like a piston pump, except it flexes a flexible diaphragm back and forth, so as to compress the space on the other side of it and produce a pumping action in conjunction with check valves. They care less about what goes through them, than a piston pump, because they require no lubrication and the vapors don't foul their crank cases.