Ever asked yourself exactly what are the Alkanes, Alcohols, and Ethers commonly used for cannabis extraction and alchemy made of? Fortunately, one doesn't have to be an Organic Chemist to understand them, as they are all simple single bonded chains of carbon, hydrogen, and sometimes oxygen. It is not until we get into the Alkanes, that we have double bonds and aromatic rings to keep track of.
Organic Chemistry classes are but a faint memory, but here is simple minded recollection of how the Simple Alkanway of understanding them.
The carbon atom is capable of bonding with four other atoms, some of which can be double bonds to other carbon atoms.
The hydrogen atom is capable of bonding with only one other atom and the oxygen atom is capable of two bonds.
Simple Alkanes:
The Simple Alkanes contain only carbon and hydrogen atoms and start with the shortest carbon chain of one carbon atom, with four hydrogen atoms bonded to it, which is Methane.
--H
HCH = Methane = CH4
--H
Note that the - marks are space holders for letter alignment, not bonds.
As the first four Simple Alkanes don't follow the Greek alphabet, as do Pentane and longer chain Alkanes, I use the mnemonic device, "Mary Eats Peanut Butter", to remember them.
It is also easy to keep track of the chemical formula for the Simple Alkanes, because there will always be twice as many hydrogen atoms as carbon atoms, plus the two on the ends of the chains.
The second Simple Alkane is Ethane, with two carbons and six hydrogen's.
--HH
HCCH = Ethane = C2H6
--HH
Next we have Propane, with three carbons and eight hydrogen's.
--HHH
HCCCH = Propane = C3H8
--HHH
Followed by Butane, with four carbons and ten hydrogen's.
--HHHH
HCCCCH = Butane = C4H10
--HHHH
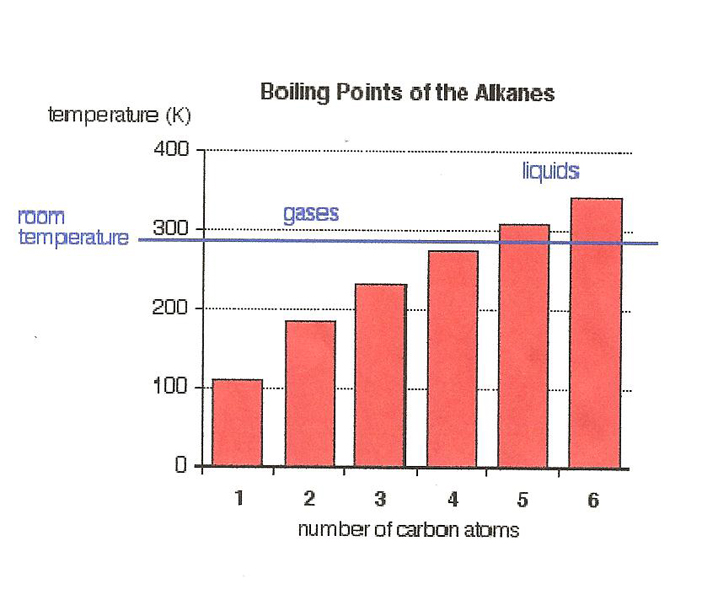
At this point, we move to the Greek alphabet with Pentane, which has five carbons and 12 hydrogen's, to Hexane with six carbons and 12 hydrogen's, and Heptane with seven carbons. There are longer chain Simple Alkanes, but they are typically not used for extraction.
Due to Vander Waal forces, as the chains get longer, their boiling points go up, as does the difficulty in purging out their residuals afterwards. Here is the general relationship of chain length to boiling points:
The Simple Alkanes are non-polar solvents, and mostly insoluble in water. Butane is the last of the Simple Alkanes that is still considered slightly water soluble, at .000325 vol/vol, or about 3.2 ml/liter.
Simple Alkanes do a good job of avoiding the polar elements, but does still extract waxes, fats, and lipids, that are non active ingredients adding to the bulk and harshness. This is easily removed by winterizing the raw oleoresin extract using a polar solvent and freezing temperatures to coagulate them, so that they can be filtered out. See page on polishing extracts for details.
The Simple Alkanes are also relatively non-toxic, with some even used in food processing. Their presence is also easily detectable by taste and smell at levels far below Threshold Limit Values, so they are a good choice for extraction. See attached MSDS sheets for details.
Simple Alkane Alcohols:
While the Simple Alkanes may be simple and dull, not so once an oxygen atom enters the picture. Depending on its location in the Simple Alkane molecule, it turns the simple hydrocarbon chain into an alcohol or an ether.
For instance Methanol, or wood alcohol, is based on the Methane molecule, with an oxygen atom bonded to one end of the chain, between the carbon and the last hydrogen.
--H
HCH = Methane = CH4
--H
--H
HCOH = Methanol =CH4O
--H
Ethanol, or grain alcohol, is based on the Ethane molecule with the oxygen located on one end, same as the Methanol.
--HH
HCCH = Ethane = C2H6
--HH
--HH
HCCOH = Ethanol =C2H6O
--HH
Propanol, or the more familiar isomer Isopropyl alcohol, is based on Propane, but the oxygen is in a different location in the isomer.
--HHH
HCCCH = Propane = C3H8
--HHH
--HHH
HCCCOH = Propanol = C3H8O
--HHH
--HHH
HCCCH = Isopropyl Alcohol =C3H8O (isomer of propanol)
--HOH
Butyl Alcohol is based on the Butane molecule, but in addition to an alcohol, it may also be present in an isomer, that is an ether, because of the oxygen atoms location.
--HHHH
HCCCCH = Butane = C4H10
--HHHH
--HHHH
HCCCCOH = Butyl Alcohol= C4H10O
--HHHH
--HH=HH
HCCOCCH = Diethyl ether = C4H10O
--HH=HH
Notice that even though it is based on butane, it is called Diethyl? That is because the oxygen atom splits the butane chain in half, which puts an ethyl chain on either side, and Di stands for two.
Of the above alcohols, Methanol, Ethanol, and Isopropyl are commonly used for polar extractions. The alcohols are more toxic than the Simple Alkanes that they are based on, but still mildly so in the minute quantities that may be present as residual solvent.
Methanol is converted into formic acid and formaldehyde by our bodies, which attack the central nervous system, particularly the optic system, which is where the stories of going blind came from.
Isopropyl similarly attacks the auditory system in sufficient concentration, so post purging is more of an issue with Methanol and Isopropyl alcohols, than with Ethanol. Not that Ethanol isn't toxic, it just takes more of it and it doesn't attack our optical or auditory systems.
The thing to keep in perspective with any substance, is that the poison is in the dosage. Higher than 75% highly salubrious and wholesome oxygen in the atmosphere will kill us dead. Everything falls into that category, it just takes more of some than others and some have other issues, like being a carcinogen or a teratogen (birth defects).
The following are the MSDS Sheets for the above chemicals, which I always advise you to check for the specific manufacturer of your solvent, because they are not always the same across the industry.
Methane Methane MSDS
Methanol Methanol MSDS
Ethane Ethane MSDS
Ethanol Ethanol MSDS
Propane Propane MSDS
Isopropyl Isopropyl MSDS
Butane N-Butane MSDS
Butyl alcohol Butyl alcohol MSDS
Diethyl ether Diethylether MSDS
Hexane N-Hexane MSDS
Heptane N-Heptane MSDS
Good MSDS source: http://www.airgas.com/content/msds.aspx





Whatsapp: +195.464.18238 Open link https://sites.google.com/view/nembutal-pentobarbital-sodiums Buy Xanax bars , Lean syrup , Oxycodone , Adderall , Percocet , Methadone , Ecstasy , Lsd and acid , Actavis Promethazine Cough Syrup , Viagra , Vyvanse , Klonopin , Fentanyl , Rohypnol (Roofies) , Ketamine , Hydrocodone 10/325, MDMA (molly) crystal and pill form , Crack Cocaine , Heroin (white, brown and tar) Weed , Marijuana , Cannabis buy in Switzerland , Germany , Spain , Italy , Strawberry , Sour Diesel , Jack Herer , Durban Poison , Haze, Pineapple Express , Blue Dream , Purple Haze , AK-47 , Grapefruit , White widow , OG Kush , Purple Kush , Black weed , CBD Oil , Hemp oil , Moonrock for sale in Switzerland , Germany , Spain , Italy ,E-mail: danny@ doctor. com https://onlinecannamedshop.com
Hello. Which would you recommend is best for extractions? Wheat, Cane or corn alcohol?
190 proof from any of those sources works equally as well. GW
[…] Alkanes, Alcohols, and Ethers | Skunk Pharm … – Ever asked yourself exactly what are the Alkanes, Alcohols, and Ethers commonly used for cannabis extraction and alchemy made of? Fortunately, one doesn’t have to … […]
When I try to winterize my run turns to complete gelatin (won't pour). Just wondering what I'm doing wrong. Has this happened to anyone and what should I be doing? Using an extractor (bho) and everclear.
Howdy, I was looking for a good solvent to make RSO in a megahome style distiller designed for alcohol. How does this solvent sound for this type of project? http://www.sciencecompany.com/-P16859.aspx?utm_source=google&utm_medium=shop&utm_campaign=prod&utm_content=NC-12886&gclid=CNPWqp267sACFQiDfgodMWwA3g Thanks Pete
dont do it bro.. not safe... just evaporate it and eat the money.
Hello...thanks for all you Folks do!.... What do you recommend for winterizing? I have used Everclear but it is to expensive. Can you all provide any less expensive options to Everclear?.. Thanks!
Methanol works better than ethanol. Check out the MSDS before using. http://www.sciencelab.com/msds.php?msdsId=9924648
There's a solvent that I've been wanting to try out lately but I don't have anything close to a closed loop system. The solvent I'm talking about is DiMethyl Ether, it is the ether of methanol and it is mostly used in the extraction of proteins from meat products for use in the food industry. It's toxicity is comparable to butane and its boiling point is -11 degrees Fahrenheit so it's got some good qualities as a solvent, the polar affinity of an ether with a very low boiling point making it easy to purge from extracts at lower temperatures meaning less degradation and evaporstion of terpenes. The best way I can think of to use this solvent is during a winterization procedure, after removing the fats, where it would be employed to make the extract much more viscous in an attempt to make the acetone-extract mixture easier to evaporate at lower temperatures to result in a finished absolute which still contains tons of terpenes. Think it over and tell me if its a solid idea or if I'm dead wrong.
Correction: Diethyl has evil spirits in it, not Dimethyl. We have almost assemble a test unit to attempt extraction and recycle using dimethyl ether.
Love this site it is the best ever, i make butane hash oil using fresh frozen material and then winterize, I have winterized with many different solvents but I have produced the most true fullmelt and terpene intact absolute using 99.9%virgin acetone! it seems to be very easy to remove the residual solvent under vacuum. I would love to hear your thoughts on this method. I've also been able to more consistently produce shatter absolutes. I also make rick Simpson oil with the acetone and have had very potent and clean oil if the solvent and material is frozen. Also I read that residual acetone is broken down in the body and safely processed into glucose while residual isopropyl is oxidized in the body to acetone. So much respect for this website you guys rock, please give me your input on these methods
We don't use acetone, because it is a known female reproductive system toxin. http://www.sciencelab.com/msds.php?msdsId=9927062
With regards to Methanol, would VP Racing Fuels: M1 (99.85% Methanol) be suitable for extractions? It costs ~$40 per 5 gallons, which as solvents go is a pretty good bang for buck.
The only MSDS sheets that I could find for VP Methanol racing fuel, says that it also includes a complex mixture of hydrocarbons, which are unidentified. Do you have an MSDS sheet for the brand you are eyeing?
www.vpracingfuels.com/download/MSDS_M1_Sep08.pdf
The MSDS doesn't show anything but methanol, with the balance ostensibly water, but as it is a fuel, rather than a reagent grade, so without further testing it is a best guess. You might pay for a GC analysis to check it for impurities yourself.
Thanks for the great input on high noon this week & also this amazing site. I have learned a lot from both. There was a question about lab versions of Naphtha. As far as I understand, in the UK at least, this is known as 'petroleum ether' or 'petroleum spirits' & is available from lab supplies companies.
Petroleum ether is primarily Pentane and Hexane and is available in the US as well. Always read the MSDS when you select a brand, as the contents are not specifically spelled out either.
Yo, that's what's up truthfully.
What about using Diethyl Ether (CAS # 60-29-7) == is the procedure the same as with butane? Soak, filter, vacuum purge, light low heat? Or is there an added step? Also will ether extract more or less of the chlorophyll -- or is it simply a function of soak time.
Butane has a dielectric constant of 1.4, Hexane a 2.0 and Diethyl ether 4.33, so it is still relatively non polar, but more polar that Butane. http://www.engineeringtoolbox.com/liquid-dielectric-constants-d_1263. The extraction technique would be more like the techniques we posted for Hexane.
Would high grade heptane grab more of the essentials than the others, being the longest chain? And for its length, it's supposedly easier on the nervous-system than hexane, how would that be? Appreciate your wisdom, js
Heptane does a nice extraction, but is even harder to purge than Hexane.
Thanks. What is the difference between "Industrial" HEXANE TO "LAB "GRADE? If the Industrial is 100% hexane not recycled,etc.
HPLC reagent grade is free enough of contaminants, that nothing shows up on a HPLC or GC. 100% seems unusually pure for an industrial grade. Do you have the MSDS for it?
no,it was picked up by someone else.
You can usually find the MSDS on line using Google.
no brand name,locally packaged
Hi,Could a person use the acetone to do a water wash like one would use hexane? As I would do a everclear wash at the end to make sure to get rid of as much acetone as possible.
It takes a non polar solvent like hexane, which is imiscible in water, so they separate. Acetone is polar, and mixes readily with water.
thanks ,I was thinking that but now I know for sure.
is denatured alcohol dangerous to use for winterization? i couldnt find the boilng point under a vacuum for the isobutyl ketone, just the atmospheric point which is at 250F! Anyhow i purged for 8 hours at 135 and it seems to be ok but i am wondering if i redisolve it in IPA will this assist in removing any more nasties? next time denatured wont be used this was an accident.
You could winterize with denatured, but not as well because most of the denaturing agents used are more aggresive a solvent than ethanol. If you are refering to Methyl isobutyl ketone (MIBK), I show a boiling point @ 760mm Hg of 115°C (239°F). There is a good nomograph for calculating boiling points at reduced vacuum: http://www.sigmaaldrich.com/chemistry/solvents/learning-center/nomo-assets.html
jobs a goodin folks Health and happiness
just Two? I have seen some of Eloquent's outstanding work. What a pair to draw to!! is it ok to link to this site from the forums? when speaking extractions? GW and skunkpharm is required reading. thanks again beans
Feel free to share any of our content on other forums. Our goal is to spread the word!
Sir!! (or Ma'am) ;) Just wanted to stop by and say hello and kudos for all that you do. Hope you all have a verrrry merry holiday Beans....
Both actually! It takes two of us guys to almost keep up with our gal, and speaking for all of us'ns, thanks for the good thoughts and hoping you have a stellar holiday too! GW
What about using Acetone or Naptha?
Acetone works, as does naphtha. Except for trying them out, we don't use either, because we don't have to and because we extract meds for other people. We stay with food grade polar solvents for oral consumption. We use Isopropyl or denatured alcohol for topicals, either which is cheaper than acetone here locally. Acetone is polar and aggressive, so freezing it and the material to -18C/0F, and pouring the acetone through the material suspended in a French Chinoise strainer, is the best method we've found. As there is interest, I will add acetone to the list of processes to document and offer on our site. "Naphtha" can mean a lot of things, and all that designation tells you, is the boiling point range of 30C to 200C, not what is in it. Even when narrowed down to "Light Naphtha", with a boiling point between 30C and 90C, it doesn't simply mean hydrocarbon chains 5 and 6 carbons long like Pentane and Hexane, it can mean any and all hydrocarbons boiling in that temperature range. If you Google Light Naphtha MSDS, and look at the different mixes that different manufacturers put in their Light Naphtha, you will see graphically what I mean. Some of those ringed molecules are on watch lists as carcinogens, mutagens, or teratogens. Both Pentane, and Hexane are both excellent choices for extraction, and a Light Naphtha mixture containing only those two constitutes would be excellent, but unless you can find such a source, that is not subject to change when the manufacturer's surplus balances shift, it is far safer to just buy Pentane or Hexane. GW
Wow, excellent response. I should have said light naptha and if it wasn't clear, in all of this I am talking about oil for internal use (ingesting). The first time I started doing this, I was using 99% iso. It worked good, but the one store that had it for sale here, stopped selling it. I was given a list (which I don't have now), of other things I could use. The only two that are readily available here are naptha and acetone. Naptha though, I have to order 45 gallon drums of it. That just isn't reasonable at the moment, so I was left with acetone. I used it and it did seem to work, although I sure didnt use your methods. I soaked the bud in the acetone for 15 minutes, strained and squished it, then added more acetone and let it soak again. I then only boiled it down once. The oli seemed fine, tasted pretty much identical to what it did when I used the ISO and seemed to have the same effects. After reading this page, I really started to wonder what the best way to use the acetone was. I will try it differently next time. I can't freeze it to -18, (unless its winter here of course), but I can stick them in our food freezer for a while first. The idea of just pouring it through the bud does worry me in terms of wasting bud, so I will likey immerse it all, but just not let it soak for more then a second or two. Should I still render it down and filter it multiple times using acetone? Also, I am under the impression that acetone leaves no traces behind, so there should be no reason I shouldnt be doing this for ingestible oil... is this right? lol
Soaking plant material in Iso or acetone for 15 minutes would produce similar results, in that they would extract a boat load of chlorophyll. The frozen material and chilled acetone pour through, is designed to reduce that. If you don't find chlorophyll objectionable, those extra steps aren't necessary. It doesn't get cold enough here either, even in winter, so we use our freezer to get down to -18C/0F. Not sure what you mean by rendering it down and filtering multiple times. Could you elaborate? All solvent extraction leaves residual solvent, though properly done, the levels are far below those of health concern. GW
On one of your pages (can't remember which one now), you talk about boiling the iso/oil down, adding more iso, freezing it, filtering it and then doing it all again... The act of boiling it down is what I meant by rendering it. Do I find the chlorophyll objectionable? I really have no clue as going by what you said, I've only had it with lots in it. If I just pour the acetone into the pail, stir it and dump it back out right away, will that make a noticable difference in the amount of chlorophyl?
You may be referring to my post on re-dissolving ISO (C3H8O) extracts in hot Ethanol (C2H6O), dropping the temperature, and filtering, to get rid of those things soluble in ISO, but not Ethanol aka EtOH. If that is the case, it gets filtered as an ISO solution, and again as an chilled -18C/0F EtOH solution. To answer your last question, yes, a soak and dump technique will work, but as you note, soak time will be a matter of seconds and gets most of it the first shot. With a pour through, you have to use a repack and do one than one extraction, to get it all. My first Acetone extraction was in a large mouth quart canning jar, using trim at outside ambient temperatures in the low 80's and with a 20 second soak and gentle shake at the start and one in the middle and end, before opening the jar and upending the contents into a wire strainer for a fast drain. After filtering the solution through a commercial restaurant sized coffee filter, I evaporated off the Acetone with a fan and no added heat, until visibly gone. It produced a dark green extract, which would not all re-dissolve in boiling 190 proof Ethanol, and after the Ethanol was chilled more material filtered out. Despite the double filtration, the solution was still dark green, as was the end product. If I were going to repeat that soak experiment, I would freeze my Acetone, and experimental stash in pint canning jars, and then give different soaking times a try. Shouldn't take many trials to narrow down 20 seconds, starting at 5 seconds. I would dry my material to around ~15% water content, and break it up loosely by hand, without creating a bunch of fines. The stuff we want, is on the outside of the plant material, so it isn't necessary to break the material up fine to increase the surface area of broken leaf margins. In point of fact, the fewer broken leaf margins the better. That is where the chlorophyll is leached out. Dumping through a wire strainer to separate the plant material from the solvent faster, and re-filtering with a paper filter, worked out well and I would do that again, The fan worked well as well for rapidly evaporating off the Acetone, but we don't live in a dusty environment. After evaporating away all visible Acetone, I would wash it out of the evaporation dish with hot ethanol and re-filter after chilling. I would then place that in a 250F hot oil bath, and boil off the ethanol, then continue until it was decarboxylated to the level I wanted, based on bubble generation. Hope that helps narrow your focus, and you have fun with your experiments! GW
Hi GW, Would this "light" Naphtha be acceptable according to the MSDS? Would you be comfortable using this? Thanks for your response http://64.26.129.203/_profiles/_msds/14-434.pdf
Fuel grade isn't something I would ingest.
Taking this further, I purchased twenty-four solvents for testing, here's a list arranged as to solvent polarity, https://www.icmag.com/ic/showpost.php?p=7933632&postcount=162 How I ordered, received, and inspected each solvent is described in detail within the thread, simply enter the solvent name in the Search this Thread box at the top of any page of the thread. Within the thread you'll also find how I primarily based my choices on the FDA USP Residual Solvent toxicity classification lists, the solvents chosen are from the Class 3 list, with a few gleaned from the Class 2 list. https://hmc.usp.org/sites/default/files/documents/HMC/GCs-Pdfs/c467.pdf
What about Ethyl Acetate? Is that not a better thc solvent than any of the Simple Alkane Alcohols?
Ostensibly so!