I have been having a discussion on another forum, on how it is possible for water to pickup chlorophyll in an extraction, when chlorophyll is basically a hydrocarbon, which is mostly insoluble in water.
I did enough research to know that I was in over my head with chemistry that I took on the late fifties and early sixties, so I asked Joe, our budding biochemist, to take a run at it.
In quick summary, before Joe's response, "define soluble?" The word soluble means different things in biochemistry, than it does in inorganic chemistry, because of the behavior of the molecules of life.
The chlorophyll molecule has a magnesium (Mg) at its rings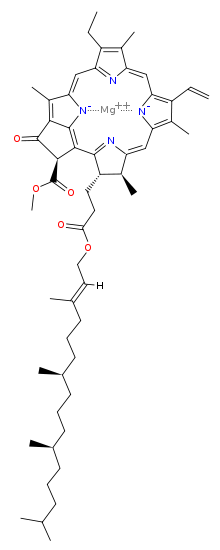 center, which makes it ionic and water-loving (hydrophilic) and a ring that is water fearing (hydrophobic) with carbonyl groups near a tail that make it polar (also hydrophilic).
For the rest of this article see: /chlorophyll-pickup-in-extractions/
center, which makes it ionic and water-loving (hydrophilic) and a ring that is water fearing (hydrophobic) with carbonyl groups near a tail that make it polar (also hydrophilic).
For the rest of this article see: /chlorophyll-pickup-in-extractions/
Sign in to set favorite





You're on the right track however you're not applying enough tactile's response to your diaphragm you going to need to amp it up by 60% . I'll be posting my design later good luck to you my friend .
To make it fullmelt bubble you should just use the static card tech or something, make something that produces a static charge across a large rectangular surface...... might be interesting. full melt apparently.
Yeah, we've played with a cassette with parchment wrapped around it, which worked and tried to use an electronic fly swatter power supply for an electrostatic charge, which didn't. Bob built a 27KDC power supply for me to use for my electrostatic precipitator experiment, which he can crank up as high as 87KDC, but it will arc about an inch per 1K volts, so close enough proximity without arcing gets to be a problem. When I wrap up the electrostatic precipitator project, I'll see what I might be able to do to produce full melt. I've always suspected that to be the foundation for Skunkman Sam's secret process, but alas, only he knows. GW
Hello world, i have been producing high quality resin from my bubble bags using a hand-held concrete vibrator that can be found online ($89-$250). the bags, ice, and h2o ratios mostly stay the same. but instead of physically crushing and tearing the material it is jolted in place, side to side, add to that, random movement, the resins spherical shape and tendency to separate by density in water, and you have some of the cleanest bubble, and higher yields due to the extended run times credited to the decreased contamination rate over time. This method is entirely scale-able using industrial plastic or stainless, conical reservoirs with a drain valve on the bottom, similar to those you would find at a farm feed supply or brewery. stainless worked better for me. put the golden h2o valve on the bottom and with out emptying your system you can drain the good stuff incrementally, over time, for quality separation. don't wait for the yellow h2o on top, you wont see it. you are NOT MIXING. The resin never moves up and around, just down. it will be waiting for you at the bottom where it belongs. oh and you hold the concrete vibrator to the side, you can experiment with various positions and mounts, however most of the yield results were similar, but holding it to the side of a stainless feed res in a walk in freezer gave this guy a yield and quality so far separated from my personal experience, it left me choice but to scream it from the highest roof top to all who would listen. I am very pleased to see the the development in this industry over the last decade, especially the focus on clean medicine. You all deserve a big pat on the back. K+ to all
Thanks for sharing Moses! The fish trap exists only because of the fish and sometimes it is possible to forget we are after the best fish, not the purdiest trap.
I'm a drum tech of 25 years I've been putting these but bumpers underneath the drummers asses and using Low frequencies . you need to get a crown 1500 and hook up to that but bumper and run it up to about half unity in utero shake that shit right clean but you better have it bolted down go find a crown amplifier 2500 from a pawnshop , you need high amplitude to make the butt bumper oscillate at the right frequency under the right load . You can look me up my name is Judd Kalsh, now let's talk about something more serious super high frequency able to loosen oil from plant matter ???
Still wondering how this project turned out. We pre-sort our plant material on large DrySiftWizard screens in order to remove as much contaminating matter (dirt, perlite, plastic debris etc) and getting decent kief, but the hands on pressure is pushing a lot of plant material through the screens.
Get yourself a ultrasonic cleaning bath, put your weed in a jar topped up with whisky, place it in the cleaning bath that is party filled with water, switch on and watch the good stuff being released from the plant material and inside few minuet's you have extracted ALL that there ever was from the weed, strain and bottle...very potent shit
Is the whisky serving as a relatively pure ethanal solvent? After straining, do you do anything to concentrate the extract further? How solid does it get? What is your whisky to material ratio? (sorry for so many questions, but I really like the idea)
If i'm not mistaken, The whiskey is a solvent... and also a post extraction drink! Further purification or processing isn't the idea. The ultrasonic cleaning bath is to increase the rate of solvation. U.C. baths are used to clean glassware in chemistry labs by basically jostling the heck out of the glassware in a solvent to dislodge whatever's stuck to the glass. It's the same principle here, only you'd be dislodging Cannabinoids and other soluble compounds into the Ethanol in the whiskey.
Check out this video http://io9.com/scientists-master-levitation-of-small-objects-with-soun-1492781926 Perhaps , A New project for Ultrasonic oil extraction? Using focused waves to seperate the heads from a stationary frozen bud? Or capturing a pile of fresh frozen trim material within the ultrasonic waves & then manipulating the levitating/vibrating bulk material through a screen? IDK just throwin out ideas off the top of my head since i spied this vid online.
Whooooa! Hee,hee, hee...................
yea this is what i was talking about with cynetics im sure theres a mad scientist somewhere who already has a supersonic death ray you can try out ;)
Did it melt?!
Dry sieve melts, but isn't full melt. It does retain more monoterpenes and I find it more tasty than full melt bubble though.
Dry Sieve will definitely full melt once you reach 90% plus resin head purity. I make full melt dry sieve all the time.
You can buy a two screen set on ebay. the top screen is a 140u and the bottom is a 70u. I found this super good material and a cheaper option than the other big names. I find that when you use shaking and bouncing more contaminate will come though with the headies. Have you ever used any static electricity?
hah, i know who you get your ideas methods from. :) Well said, John Berfelo and Bubbleman Marc have mainstreamed a static sifting method that grabs all headdies in testing. All you need is a parchment paper wrapped around a bluray or video game box. thats it! A guy named meze on youtube showed everyone on hash church a while back, bubbleman talks about the meze's test methods in his YT video called "Bubbleman filing your dry sift quiver" -- if you are into the solventless game, dry sift or butane-less dabs, you gotta check that video out. You can purify your dry sift with a piece of static-charged parchment, basically. Explained more in vid, cheers -Derty Qwerty 42N
I had a set of 12 inch woofers and 1000 watt amp---would literally vibrate your lungs! There are CD's you can buy for "tuning" your subs. I had several. If you have a good set of comp speakers with a sub you can get some excellent vibrations from it using those CD's. A good test song is Make the Car go Boom by Magic Mic. Sometimes the frequency is so low you can hardly hear it but you surely feel it!! I'm going to try your experiment!
Have fun and I hope you will share your results!
i would use 1 really good 10 inch sub from the side at a slight angle playing sine wave tracks you don't even have to touch the speaker to the seiv just get a proper distance for your desired frequency range i used to levitate hanging objects in my car like this
Good idea!
ty :) id been thinking of it for a wile trying to acoustically break the trichome stems would be near impossible but you could tune your setup to the resonant frequency of your screen and make it do crazy things i had also been thinking of this + vacuum pump instead of a fan for agitating thin film evaporation in a semi sealed dust free environment the plates used in cymatics might be able to do better with less power than subwoofers tho
I use my cheap little orbital sander, you guys are wasting time and money with this science project
But having lots of fun!
Canadian seed maker, House of the Great Gardener, has a method using a lab shaker. The lab shaker is similar to a paint shaker except far less aggressive -- which is perfect for this process as too aggressive gets you lowered quality. He put his dual drysift screens on top of the lab shaker he found cheap used (they are expensive tools when bought new for science labs usually) Press the button, walk away for 5 or 10 minutes. It rotated the sift screens at a gentle but very constant/reliable motion because with these lab shakers there is a knob to adjust the speed to what you want it to be :) Just some ideas..things are going mega-commercial this year. Lots of people are going to lose income farming this soon, because mainstream supply is catching up to insane mainstream demand
I slowly heated my extracted matter until it was soft and whip-able. Question: will prolonged heat ruin my extraction? It was over a low heat for a few hours while i waited for it to stop bubbling. Bubbling I understood was the butane evaporating. Am I misunderstanding? I was worried about the effects of prolonged heat so I removed it.
Prolonged heat will decarboxylate the oil and change THC into CBN. Check out the thread at http://skunkpharmresearch.com/decarboxylation/. Take a look at the decarboxylation chart posted there, which will give you an idea of what to expect at different temperatures.
another emerging advancement! eagerly awaiting progress on this front (err... all SkPh projects! :-))
you guys are awesome! Great write-up.
Thanks for the good thoughts!