A brother recently posted on Facebook he had injected around 6 cases of butane into a container and when he evaporated it off to see what was left, there was a heavier residual oil that he described as smelling like a tire factory.
There are a number of possibilities offered on what it is, but no definitive analysis as yet, so we decided to run an experiment ourselves, with us in control of the variables such as cleanliness.
There are of course things that we know are in butane, such as sulfur in the low ppm to ppb range, and longer chain oleaginous waxes, which is what all the XXXXXX refining is to remove, as they clog the small orifices of expensive butane lighters.
If you concentrate enough butane, you would expect to see a measurable amount of those left behind and they may explain the Mystery Oil.
As far as the smell, tires are mostly made of synthetic rubber formulated out of styrene and butadiene, so the butane present could explain a butyl rubber odor, if mixed with sulfur and longer chain alkane waxes.
All conjecture of course, so until we can get a clean sample into a lab for a HPLC/MS analysis, all speculation is conjecture.
I tried to conduct the experiment yesterday, but alas we only had one case of butane on hand and the wholesale house was closed. One 12 can case turned out not to be enough, so I will repeat today.
I ran the one case of Lucienne through a brand new cold trap that I assembled out of new Mk III components, kindly loaned to us by Specialized Formulations, so that I had a pristine and uncontaminated unit for the tests. To remove any existing contamination beforehand, I first boiled in hot soapy water and wiped down with 190 proof ethanol. I did get some light oil from this trial, but not enough to filter and send out for analysis.
I say filter, because the first thing that I saw was paint chips from the outside of the can in the oil. What a tasty thought for those who can tap their butane and don't subsequently clean the pot before running, or winterize or filter the oil.
While the sample was larger than the .05 grams needed for GC, it was small and full of paint chips, so I decided to rerun the experiment with more butane and add a coffee filter section to my test sled.
The oil didn't smell like an old tire factory, but like a light petroleum fraction (distillant), with a hint of butane odor, so the tire factory odor may come with a larger sample.
It readily dissolved in 190 proof ethanol.
Ethanol is a simple alkane alcohol, and simple alkane hydrocarbons do readily dissolve in it.
Just to make sure my memory was correct, I dumped some hexane in the beaker of mystery oil and ethanol, to verify that it did mix, because all alcohols don't mix with simple alkanes like hexane. It did.
I also cut apart all of the cans and felt inside for lubricity. There was none.
I inspected all the welds for quality and the welds were all sound and unoxidized.
I also cut apart the valve to better understand why lubricant would be required. It is is a simply made plug valve and needs no lubricant.
PS: Don't cry ya'll! The spill in the picture of alcohol is actually water from taking the cold trap out of the hot pot, not $37/1.75L 190 proof.
Today I will pick up a long case of Lucienne and try again.
8-27-2013
Well, 72 cans were enough to get a good sample and I will drop it off at a HPLC/MS lab today. I added a filter section this time, so the sample was paint chip free and appears to be a light petroleum fraction, with a light petroleum odor. There was no tire factory odor, nor the smell of sulfur at this concentration level and I will refrain from conjecture until I see the analysis.
9-4-2013
OK, I picked up the test results and am still going through it a line at a time, pulling up the MSDS sheets, starting with the items of concern.
The first thing to keep in perspective, is that the total oil was 12 ppm from 21.3 liters, so the PPM in the study should be divided by 1,000,000 and multiplied by .000,012, to get the concentration.
That concentration number X 1,000,000 will give you parts per millionth.
In summary, there were simple Alkanes present as long as C-16, which are not of health concern at the levels present.
In addition there are aromatic Alkenes present, that are of serious concern, but not at levels remotely close to exposure limits.
The concern with those Alkenes is really not reaching toxic limits, but the fact that some are known carcinogens, mutagens, teratogens, etc.
IE: Cyclohexane, isocyanato, which most likely came from the gaskets and seals used in the cans and process, because it isn't found naturally in butane.
Nasty bad shit, with an no exposure limits established and an intravenous LD-50 mouse of 13 ppm and present at the level of .000000001728 concentration, or .001728 parts per millionth. That is about 7500 times lower than the LD-50.
Another bad nasty present was Benzenamine, 3,5, at the levels of .035778 parts per billionth, with a Permissible Exposure Limit of 2 ppm TWA for 8 hours. That is 56000 times lower than Permissible Exposure Limits.
Benzene 1,1 showed up at a concentration of .000000003678, or .000003678 PPM, with a Permissible Exposure Limit of 110 ppm TWA 8 hours.
I could go on, but it will take me awhile to pull MSDS sheets for the whole list, so I've attached the raw data. Sorry the quality isn't better, but the original is barely readable and this was copied at 1200 bit resolution
Even if nothing is present at levels of serious health concern, I would prefer to not have any of that stuff in there, so in addition to fractional distillation using vacuum, we will be experimenting with Bentonite filters.
Here is the parts per billionth summary: You will note that there are redacted lines of scary stuff. Those were redacted by the lab itself, as it is their carrier solvents used for the tests and were not present in the original sample.
9-11-2013
I'm not clever enough to outsmart this edit program, and it continues to thwart my adding anything at the end, so here it is in the middle, and I'll let Joe sort it out later.
This experiment was designed to determine the contents of the mystery oil to the ninth decimal place, and I believe has accurately done so.
It was not designed to accurately determine the weight to the ninth decimal place, so that will be the next round of experiments. For that purpose, I've ordered a fresh 50# refrigerant tank, as all of ours have had both n-Butane and lighter butane in them, so can't be relied upon to be un-comtaminated themselves.
I've decided to rely on a third party lab to determine exact weights, after which we will have our in house chemistry brain trust The lab technician that I work with, is on vacation and I'm fixing to be, so it may take a couple weeks to come up with a formal plan.
My intent is to determine actual total ppm as compared to advertized ppm, and infer that the components are the same as the first detailed sample.
Readers have commented on having found more oil in other brands, and even in the same Lucienne brand that I tested, at levels that are triple the 50ppm limits they are certified to contain. If that is the case, I plan to make that information public and a third party documentation will be cleaner and come in handy in case of lawsuits.
It would be prohibitively expensive to test all butane sources, but we will test the half a dozen or so primary ones used for extraction, including Airgas, Praxair, and Matheson.
I've also picked up a couple of cases of specialty butane typically used for cooking, at the suggestion of Skyhighler, and will be fractionally distilling them, to see what the relative oil content is and if mercaptans are detectable.
A point that I hope ya'll keep in perspective, and that is that even at the higher ppm that others are discovering the oil, the ppm of the evil spirits in the finished product, would still be under limits set by NIOSH.
Having said that, as I would personally rather not have them there at all, we will hence forth vacuum distill all butane, regardless of source, before using it in our extraction process.
9-10-2013
It is taking awhile to sort through to these items, so for those of you who wanted the complete names and a more legible copy, here are the legible names and CAS#'s at the bottom of the page:
9-23-2013
OK, I dropped off a new sample of Lucienne in the can at Specialty Analytical, as well as two cans each of Skyhighler's Iwatani and Gasone brands for ppm testing. I expect the results by Friday, which will be third party and should cut through any concerns about my accuracy.
Cost per run was $75/ea, so I limited it to these three brands, with double tests on the Lucienne. We will test other brands of interest at a later date, after we see what we get with these tests.
I discharged a can of Gasone and Iwatani into a bain marie for a sniff test, and they both definitely have mercaptans added, so even if they are ultra low residual, our sensory threshold for ethyl mercaptan is 2.8 parts per billionth., and the final oil would taste and smell of it.
Damn nice flame though! I also ordered a Iwatani creme brulee torch head on sale, and it works better than my propane plumbers torch.
Hee, hee, hee thanks Skyhighler for setting me up with a slick new dabbing torch and more than a years supply of butane for it!
Although I wasn't able to get the same lot of Lucienne that I tested before, because I picked up the last 6 pack at the distributor, I was able to get two different lots of Lucienne from a store shelf, so we can test for control. If they are all significantly different, it will support an out of control process theory.
I also talked to Marty about how we could further refine the tests on the items of interest, specifically just the compounds that are considered evil spirits, despite their low levels. He is reviewing the list and costs for individual standards and we will discuss further later this week.
Still nothing on the list remotely close to established permissible exposure levels, so far thousands or millions of times below if calculated for the residuals in the extracted oil itself, but still looking and listening for input.
More to be revealed.......................
9-23-2013
As ya'll may have noticed, there is considerable controversy on this project, so I would like to put the conflict in perspective, as I see it.
We analyzed a sample of the residual oil from butane fractional distillation to the Parts Per Billion level and have a GC/MS printout. That means that in addition to retention time in the GC column, we have the component peaks from the mass spectrometer.
The argument has been made that isn't accurate enough, even though the residuals of the evil spirits in the extracted oil, would be thousands to millions of times below published permissible exposure levels.
It is absolutely true that we can buy individual standards and refine our breakdown of the peaks of aromatics, where there are minute differences in the molecule, but surprises on the order of 1000, or 1,000,000 are highly unlikely.
We are more likely to find that although some of the benzene peaks on the parts per million list, do break down into other benzene compounds, not all benzene compounds are listed as a carcinogen, so the levels in the oil may actually be reduced further.
We are continuing to work with Specialty Analysis to identify compounds worthy of refined examination, and they've expressed special interest in this project, as well as willingness to purchase the individual standards for evil spirits in sufficient quantity to bear closer examination.
The project is advancing in an orderly manner, without MO Jack's participation, and the results will be all third party from a certified forensic lab, so I will be just another spectator and hopefully above reproach this time.
I disconnected communications with MO Jack, because the hoopla was greater than the project itself, and he was becoming confrontational. He seemed fine with questioning my empirical results, but not with me questioning him.
Some of his statements were also directly refuted by the individuals whom he quotes, leaving me to choose between their statements and his.
I also found bits and pieces of my statements on other forums, without the rest of my qualifying statement, which I consider to be stacking the deck and misleading.
If you read the comments column, you will also find that he has a SCFE CO2 system on order from Eden Labs, and will be in direct competition with BHO extraction, so I have other reasons to be concerned about his impartiality.
Regardless whether those issues are my error or not, there is no question that we can resolve what is in the butane without spending an even greater amount of time nit picking details at the parts per trillion levels and bickering amongst ourselves.
Best wishes to MO Jack with his own experiments, wherever they may lead him.
GW
10-3-13
Still awaiting lab results for the last three, but did get the results for two different lots of Lucienne, as well as Gasone and Iwatani.
The nice lady in the office and I miss-communicated earlier in the week, and I incorrectly reported the last Iwatani results as Lucienne. Even the ppm is incorrect, as the Iwatana cans are 394 ml instead of 300 ml.
That aside, and cutting to the chase, here are the correct Parts Per Millionth residual contamination, after evaporating away the butane, as reported by a certified third party analytical lab, for the first three brands.
The balance of the information on other brands to follow, ostensibly Monday:
On the subject of refining our search, Marty ordered the standards required to further investigate the aromatics detected in the ppm range, as well as the cyanide compound in the ppb range. More on that after the standards arrive and more experiments are conducted.
10-4-13
RESIDUAL PPM CONTAMINANTS IN BUTANE BY BRAND from certified 3rd party lab
|
NO |
Brand |
Volume |
Residuals |
Percent |
PPM |
|
1 |
Lucienne |
300 ml/173 gm |
.24 mg |
.000,001,387 |
1.4 |
|
2 |
Lucienne |
300 ml/173 gm |
.18 mg |
.000,001,040 |
1 |
|
3 |
Gasone |
394 ml/227gm |
.14 mg |
.000,000,662 |
.66 |
|
4 |
Iwatani |
394 ml /227 gm |
.41 mg |
.000,001,806 |
1.8 |
|
5 |
Vector |
320 ml/184 gm |
|||
|
6 |
Powers |
300 ml/173 gm |
|||
|
7 |
Newport |
300 ml/173 gm |
10-7-13
The moment some of ya'll have been waiting for has arrived, with the actual ppm residual contaminants in butane, by some of the common brands, as well as a couple not commonly used for butane extraction for comparison.
No hoopla or fanfaronade, just the actual ppm residuals by brand, as measured by a certified third party analytical lab.
Note that the ppm in the Gasone and Iwatani include Thiol mercaptans for leak detection. Thiols are alcohol analogs, where the oxygen atom is replaced by a sulfur atom and adds a garlic odor.
Note that the highest ppm found in any brand was 7% of their certified maximum of 50 ppm.
Also note that except for Lucienne, these were single can grab samples, and Lucienne is only two different lots, so the differences between brands, especially by the same refiner, may even out. IE: Lucienne and Newport.
They may not too, as the same refiner doesn't necessarily mean the same refinery, which could also account for the differences.
RESIDUAL PPM CONTAMINANTS IN BUTANE BY BRAND
|
NO |
Brand |
Volume |
Residuals |
Percent |
PPM |
|
1 |
Lucienne |
300 ml/173 gm |
.24 mg |
.000,001,387 |
1.4 |
|
2 |
Lucienne |
300 ml/173 gm |
.18 mg |
.000,001,040 |
1 |
|
3 |
*Gasone |
394 ml/227gm |
.14 mg |
.000,000,662 |
.66 |
|
4 |
*Iwatani |
394 ml /227 gm |
.41 mg |
.000,001,806 |
1.8 |
|
5 |
Vector |
320 ml/184 gm |
.51 mg |
.000,002,771 |
2.8 |
|
6 |
Powers |
300 ml/173 gm |
.34 mg |
.000,001,965 |
2 |
|
7 |
Newport |
300 ml/173 gm |
.61 mg |
.000,003,526 |
3.5 |
* Contains Mercaptans
So now that we have two bits of empirical scientific data, derived by a certified third party lab, instead of the anecdotal information on Facebook and the web that we started with, lets do the math to put this in perspective.
The previous part per billionth analysis showed 1,4 Dichlorobenzene at a combined total level of 55 parts per billionth, or .000,000,055. We all agree that we want no part of 1,4 Dichlorozenzene, because it has been declared a carcinogen, and given a low TWA CEIL of 110ppm by NIOSH.
http://www.sciencelab.com/msds.php?msdsId=9923722
55 parts per billionth in the concentrated residual itself, is 2000 times smaller than 110 parts per millionth TWA Ceiling imposed by NIOSH.
It will in addition be further diluted in an extract, considering that at the worse case total residual contaminant found was 3.5 ppm, besides being dilute in the extracted oil itself. Lets look at that math.
.000,000, 055 X .000, 0035 = .000,000,000,000,192 or 200 parts per quadrillion in the butane used for extraction.
Assuming a 40 gram trim extraction, using 300 ml of butane, and yielding only 10%, 300 ml butane would deposit .000,000,000,058 ml of 1,4 Dichlorbenzene in 4 grams of concentrate.
.000, 000, 000, 058 ml X 1.2475 gms per ml = .000,000,000,072 grams of 1,4 Dichlorobenzene in 4 grams of concentrate.
.000,000.000,072 grams divided by 4 grams =.000,000,000,018 or 18 parts per trillion.
110 ppm TWA Ceiling (.000, 110) divided by residual 1,4 Dichlorobenzene level of 18 parts per trillion (.000,000,000,018) = 6,111,111 or about one six millionth (1/6,000,000th) of maximum allowable exposure level.
4 grams of oil will produce about 20 200 mg hits, so each hit would be about 1/20th of 1/6,000, 000, so exposure per hit would be about 1/1,200,000,000 of the 110 ppm maximum.
Soooo, now that we have put things in perspective, where do we go from here?
We are planning some more testing of different brands and sources and further refined testing of all the evil spirits, but we have come far enough to know that while we would prefer to not have the unwanted contaminants in our butane, its presence is thousands, or even billions of times below published levels of concerns by health professionals.
We’ve also learned that it is easy to remove, using a cold trap and a refrigerant recovery pump, so those of you with the required equipment can take it out, making the point moot.
That is what we now do, because we can, but continue to use the oil we produced before we started fractionally distilling the butane.
10-23-13
The Colibri and Ronson sample results arrived and are as per the following chart:
Given the price and hoopla, I expected the Colibri to be lower. I wonder if it is the same product, after the company was bought out?
To consider, is that except for 2 sample lots of Luciene, all of these are single grab samples, so we don’t know what the median, mode, mean or standard deviation is.
More later, after we test Airgas, Matheson, and Praxair.
The lab asked for more time on the detailed component analysis, as well as the followup experiments for MO, to attempt to reconcile the differences between his PPM rates and theirs. More as that develops.
The good news, is that they’ve agreed to start running cannabis samples, and have more toys than any of the local cannabis labs, such as GC, MS, HPLC, etc.
RESIDUAL PPM CONTAMINANTS IN BUTANE BY BRAND
|
NO |
Brand |
Volume |
Residuals |
Percent |
PPM |
|
1 |
Lucienne |
300 ml/173 gm |
.24 mg |
.000,001,387 |
1.4 |
|
2 |
Lucienne |
300 ml/173 gm |
.18 mg |
.000,001,040 |
1 |
|
3 |
*Gasone |
394 ml/227gm |
.14 mg |
.000,000,662 |
.66 |
|
4 |
*Iwatani |
394 ml /227 gm |
.41 mg |
.000,001,806 |
1.8 |
|
5 |
Vector |
320 ml/184 gm |
.51 mg |
.000,002,771 |
2.8 |
|
6 |
Powers |
300 ml/173 gm |
.34 mg |
.000,001,965 |
2 |
|
7 |
Newport |
300 ml/173 gm |
.61 mg |
.000,003,526 |
3.5 |
|
8 |
Colibri |
270ml/156 gm |
2.34 mg |
.000015 |
15 |
|
9 |
Ronson |
330 ml/190 grams |
42.7 mg |
.000224 |
224 |
* Contains Mercaptans
1-18-2014
Here are some residual test results conducted by Skyhighler and posted on Toke City at :
and best to worst at:
https://www.icmag.com/ic/showpost.php?p=6099306&postcount=28
Power (0X) had 0.021g of residue
Power (0X), Korea, 300ml can, reads on the bottom “130819”
Power 5X #1 had 0.02g of residue
Power 5X, Korea, 300ml can, reads on the bottom “130820”
Power 5X #2 had 0.019g of residue
Power 5X, Korea, 300ml can, reads on the bottom “130731”
Power 7X had 0.029g of residue
Power 7X, Korea, 300ml can, reads on the bottom “120716”
Newport #1 had 0.01g of residue
Newport, England, 300ml can, reads on the bottom “DOM 24.04.13 20:33”
Newport #2 had 0.004g of residue
Newport, England, 300ml can, reads on the bottom “DOM 26.03.13 19:09”
Vector #1 had 0.03g of residue
Vector, Korea, 320ml can, reads on the bottom “AUTHENTIC VECTOR GAS 2013.04.06”
Vector #2 had 0.02g of residue
Vector, Korea, 320ml can, reads on the bottom “AUTHENTIC VECTOR GAS 2013.04.06”
Vector #3 had 0.028g of residue
Vector, Korea, 320ml can, reads on the bottom “AUTHENTIC VECTOR GAS 2013.04.06”
Vector #4 had 0.028g of residue
Vector, Korea, 320ml can, reads on the bottom “AUTHENTIC VECTOR GAS 2013.04.06”
Vector 14X #1 had 0.019g of residue
Vector 14X, Korea, 320ml can, reads on the bottom “AUTHENTIC VECTOR GAS 2013 09 10”
Vector 14X #2 had 0.018g of residue
Vector 14X, Korea, 320ml can, reads on the bottom “AUTHENTIC VECTOR GAS 2013 09 10”
Lucienne #1 had 0.03g of residue
Lucienne, England, 300ml can, reads on the bottom “DOM 16.07.13 13:28”
Lucienne #2 had 0.002g of residue
Lucienne, England, 300ml can, reads on the bottom “DOM 16.07.13 13:28”
Lucienne #3 had 0.001g of residue
Lucienne, England, 300ml can, reads on the bottom “DOM 16.07.13 13:28”
Lucienne #4 had <0.001g residue
Lucienne, England, 300ml can, reads on the bottom “DOM 16.07.13 13:28”
Ronson #1 had 0.01g of residue
Ronson, USA. 300ml/165g can, reads on the bottom “11713”
Ronson #2 had 0.001g residue
Ronson, USA. 300ml/165g can, reads on the bottom “11713”
Ronson #3 had <.001g residue
Ronson, USA, 300ml/165g can, reads on the bottom “11713”
Spark 7x had 0.04g of residue
Spark 7x, Korea, 300ml can, reads on the bottom “120404”
King had 0.033g of residue
King, Korea, 300ml can, reads on the bottom “12.04.25”
Fasfil 5X #1 had 0.030g of residue
Fasfil 5X, Korea, 300ml can, reads on the bottom “120718”
Fasfil 5X #2 had 0.032g of residue
Fasfil 5X, Korea, 300ml can, reads on the bottom “120718”
whip-it! 9X had 0.025g of residue
whip-it! 9X, Korea, 320ml can, reads on the bottom “130708”
whip-it! Premium had 0.003g of residue
whip-it! Premium, UK, 400ml can, reads on the bottom “239 M 17:25 1”
Neon #1 had 0.004g of residue
Neon, China, 300ml can, reads on the bottom “Manufactured On 20/12/2012/ (00488)” (no added odorant/mercaptan)
Neon #2 had 0.005g of residue
Neon, China, 300ml can, reads on the bottom “Manufactured On 20.02.1200413” (unusable due to added odorant/mercaptan)
Neon #3 had 0.001g of residue
Neon, China, 300ml can, reads on the bottom “Manufactured On 25/05/2012(00446)” (no added odorant/mercaptan)
Neon 5X #1 had 0.001g of residue
Neon 5X, China, 300ml can, reads on the bottom “Manufactured On 20/05/2013(00506)” (no added odorant/mercaptan)
Neon 5X #2 had 0.015g of residue (stinks, odorant/mercaptan?)
Neon 5X, China, 300ml can, reads on the bottom “Manufactured On 11/05/2012(004236)”
Neon 5X #3 had 0.004g of residue
Neon 5X, China, 300ml can, reads on the bottom “Manufactured On 25/5/2013(00505) (no added odorant/mercaptan)
Lava had 0.033g of residue
Lava, Korea, 5.3oz/150g can, reads on the bottom “070321”
Zippo had 0.001g of residue
Zippo, USA, 5.82oz/165g can, reads on the bottom “H1513”
Colibri had 0.001g of residue
Colibri, UK, 300ml can, reads on the bottom “DOM 18.04.13 06:35”
Stok FYR had 0.023g of residue
Stok FYR, UK, 5.8oz/165g can, reads on the bottom “DOM 07.08.13 15:57”
Iolite had 0.010g of residue
Iolite, England, 300ml can, reads on the bottom “DOM 30.07.13 22:45”
Gasone 5X had 0.028g of residue
Gasone 5X, Korea, 5.8oz/165g can, reads on the bottom “130511”
Xikar #1 had 0.010g of residue (chemical smell, avoid)
Xikar, USA, 8oz/225g can, reads on the bottom “CC26/0947”
Xikar #2 had 0.007g of residue (chemical smell, avoid)
Xikar, USA, 8oz/225g can, reads on the bottom “CC26/1153”
Clipper 7X had 0.004g of residue
Clipper 7X, China, 4.89oz/139g can, reads on the bottom “26.03.2012”
Lotus had 0.002g of residue
Lotus, England, 400ml/13.4oz/222g can, reads on the bottom “DOM 03.10.12 14:03”
Jetline had 0.027g of residue
Jetline, Korea, 330ml can, reads on the bottom “130410”
Capital N-butane #1 had 0.008g of residue
Capital N-butane, USA, 6.6oz/187g can, reads on the bottom “13337”
Capital N-butane #2 had 0.009g of residue
Capital N-butane, USA, 6.6oz/187g can, reads on the bottom “13337”
Puretane #1 had 0.006g of residue
Puretane, USA, 300ml/167g can, reads on the bottom “13339 (1) 34623 06332”
Puretane #2 had 0.006g of residue
Puretane, USA, 300ml/167g can, reads on the bottom “13339 (1) 34623 06324”
Clipper 12X had 0.025g of residue
Clipper 12X, Spain, 170g can, reads on the bottom “QT31E”
Comoy’s had 0.015g of residue
Comoy’s, UK, 300ml can, reads on the bottom “DOM 23.05.13 05:28”
Smoke It’s had 0.007g of residue (stinks!!! don’t use!)
Smoke It’s, 300ml with no country or date of manufacture on the can
Meteor 7X had 0.030g of residue
Meteor 7X, Korea?, 165g can, reads on the bottom “130517”
Cloud 9X had 0.003g of residue
Cloud 9X, 300ml can with no country or date of manufacture
Iwatani #1 had 0.03g of residue
Iwatiani butane fuel, Korea, eight ounce can, reads near the top of the can “130506” (unusable due to added odorant/mercaptan)
Iwatani #2 had 0.010g of residue
Iwatiani butane fuel, Korea, eight ounce can, reads near the top of the can “130506” (unusable due to added odorant/mercaptan)
Those were my results, here’s jackgastche’s:
Vector #1 had 0.04g of residue
Vector, Korea, 320ml can, reads on the bottom “5/12/2012”
Vector #2 had 0.04g of residue
Vector, Korea, 320ml can, reads on the bottom “5/12/2012″
Vector #3 had 0.06g of residue
Vector, Korea, 320ml can, reads on the bottom 5/12/2012″”
Vector #4 had 0.03g of residue
Vector, Korea, 320ml can, reads on the bottom “5/13/2013”
Vector #5 had 0.04g of residue
Vector, Korea, 320ml can, reads on the bottom “5/13/2013”
Vector #6 had 0.04g of residue
Vector, Korea, 320ml can, reads on the bottom “5/13/2013”
Vector #7 had 0.02g of residue
Vector, Korea, 320ml can, reads on the bottom “12/15/2011”
Vector #8 had 0.02g of residue
Vector, Korea, 320ml can, reads on the bottom “12/15/2011”
Vector #9 had 0.03g of residue
Vector, Korea, 320ml can, reads on the bottom “12/15/2011”
Colibri #1 had 0.03g of residue
Colibri, UK, 300ml can, reads on the bottom “8/16/2011”
Colibri #2 had 0.03g of residue
Colibri, UK, 300ml can, reads on the bottom “8/16/2011”
Colibri #3 had 0.03g of residue
Colibri, UK, 300ml can, reads on the bottom “8/16/2011”
Xikar #1 had 0.002g of residue (chemical smell, avoid)
Xikar, UK, 400ml can, reads on the bottom “3/13/’12”
Xikar #2 had 0.002g of residue (chemical smell, avoid)
Xikar, UK, 400ml can, reads on the bottom “3/13/’12”
Xikar #3 had 0.002g of residue (chemical smell, avoid)
Xikar, UK, 400ml can, reads on the bottom “3/13/’12”
Xikar #4 had 0.003g of residue (chemical smell, avoid)
Xikar, UK, 400ml can, reads on the bottom “3/13/’12”
Xikar #5 had 0.003g of residue (chemical smell, avoid)
Xikar, UK, 400ml can, reads on the bottom “3/13/’12”
My first tests were weighed with this inexpensive .01 gradient scale,
http://www.amazon.com/American-Weigh…ords=.01+scale
My results to the .001 digit were weighed with this inexpensive .001 gradient scale,
http://www.amazon.com/gp/product/B00…?ie=UTF8&psc=1
The results from jackgastche were weighed with a .001 gradient scale, but rounded off to the .01 digit.
How the test was done,
http://www.tokecity.com/forums/showt…=1#post1334342
Best to Worst list extrapolated from the above results,
http://www.tokecity.com/forums/showt…=1#post1338141
1-29-2015
Mo continues to exploit this issue on facebook, and when I posted the following post, putting things in perspective, which resulted in an attack on the messenger, so I am posting it again here.
Cutting to the chase, define zero!
Do you cut off at percent, parts per millionth, parts per billionth, parts per quadrillian???????
In chemistry, and in toxity, there is no finite zero and if you analyze mothers milk or drinking water, you will find things like gold, arsenic, etc, at the parts per billionth level.
Poison is in the dosage, and even a 75% oxygen atmosphere will kill us mortals.
So, knowing that, NIOSH government hygienist studied the relative toxicity of the different chemicals that end up in our bodies, and set maximum standards for exposure.
In addition, the FDA has set standards for what is allowable in food products and pharmaceuticals.
We are not pilgrims in a strange land, professionals have plowed this ground before those of without advanced degrees in the medical field.
We also have GC/MS analysis measured in Parts Per Billionth by a certified third party forensic lab, telling us precisely what is in the butane, making the claims that butane has a lubricant added for lighters ludicrous, and continuing to perpetuate the myth after the empirical results were made readily available, suggest either ignorance or secondary motives.
No wild ass guessing necessary, those actual results are may be read at:
/bho-mystery-oil/
What is removed in a single pass depends on the temperature and pressures used.
For instance, the most common residual found if the distiller elevates the temperature too high, or pulls a vacuum, is Pentane.
Pentane boils at 33.1C/97F under one atmosphere pressure. We use an 29C/85F and stop recovering at zero gauge/atmospheric pressure. Distilling hotter and recovering below 0 gauge doesn’t leave the Pentane behind.
http://www.sciencelab.com/msds.php?msdsId=9927384
Do we eliminate all the Pentane? No we don’t reduce the Pentane to absolute zero.
What we do is lower it below the parts per millionth level, and into the parts per billionth range.
What does that mean health wise? The FDA/Pharma standards say:
http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm073395.pdf
Solvents in Class 3 (Table 3) may be regarded as less toxic and of lower risk to human health. Class 3 includes no solvent known as a human health hazard at levels normally accepted in pharmaceuticals.
However, there are no long-term toxicity or carcinogenicity studies for many of the solvents in Class 3. Available data indicate that they are less toxic in acute or short-term studies and negative in genotoxicity studies.
It is considered that amounts of these residual solvents of 50 mg per day or less (corresponding to 5,000 ppm or 0.5 percent under Option 1) would be acceptable without justification. Higher amounts may also be acceptable provided they are realistic in relation to manufacturing capability and good manufacturing practice (GMP).
Ya’ll will note that 5000 ppm, is several orders of magnitude above most lighter brands that have not yet been cleaned.
You may see the orders of magnitude found in the butane I had tested, as well as see how that ranks them compares to others tested by 3rd party individuals like Skyhighler:
To keep toxicity in perspective, please note that Class 3 chemicals includes solvents used as food stuff, like Ethanol, and Acetic acid (vinegar).
That brings us back to the subject of perspective!
How much concentrate would a 1 gram per day cancer patient ingest at the current dispensary standards of 50 ppm, certified by third party lab?
50mg/1000mg = .000500 or 500 ppm maximum allowable. One order of magnitude below the 5000 ppm maximum exposure.
Current standards of 50 ppm, are therefore yet another order of magnitude below that, so if it passes, it is at least two orders of magnitude less that levels of concern.
Bringing us back to the 1000 mg/day patient, whom at the 50ppm limit, would be 50ppm/1,000,000ppm = .00005. .00005 X 1000 mg=.05 mg per day exposure
.05 mg/day is three orders of magnitude less than 50 mg per day or 1/1000th.
Clearly BHO concentrates regularly pass so as to be sold in dispensaries, so messages suggesting that it is difficult to do so, are not addressing that reality.
Sooooo, how about those selling concentrates directly and not lab testing them?
Good question?? It has been an unregulated market, but you will note that that lack of regulation is history in some states and more are following.
What is the probability that some of the untested ones are above 50 ppm residual solvent?
I would venture about 100%.
Do those represent the market?
Not remotely, as demonstrated by certified third party lab results.
His response:
For the love of God! Gray Wolf is just another backyard-scientist running canned lighter fluid through HVAC recovery pumps on a picnic table in an attempt to hold on to the status-quo of profits from processes that use dirty gas. If he is your source of science, please, don’t share it here. Cheers.
Contaminant as found by Specialty Analytical using GC/MS
|
NO |
CONTAMINANT |
Cas# |
|
1.0 |
Parts Per Billionth |
|
|
1.1 |
Butanal, 3-methyl |
000590-86-3 |
|
1.2 |
Decane, 1,1, oxyblis |
002456-28-2 |
|
1.3 |
Decane |
000124-18-5 |
|
1.4 |
3-heptane,2,2,4 dimethyl |
002213-23-2 |
|
1.5 |
Sulfurous acid, cyclohexylmethyl |
1000309-22-4 |
|
1.6 |
3-heptene, 2,2,4,6,6 pentamethyl |
000123-48-8 |
|
1.7 |
1,2-Dichlorobenze-D4 |
002199.69.1 |
|
1.8 |
1,4-Dichlorbenze-D4 |
003855-82-1 |
|
1.9 |
Oxalic acid, cyclobutyl nonyl ester |
1000309-70-0 |
|
1.10 |
1-Octanol, 2-butyl |
003913-02-9 |
|
1.11 |
Oxalic acid, isobutyl octyl ester |
1000309-37-3 |
|
1.12 |
1-Bromodocosane |
006938-66-5 |
|
1.13 |
Isooctane, (ethenyloxy) |
037769-62-3 |
|
1.14 |
Oxalic acid, allyl nonyl ester |
1000309-23-7 |
|
1.14 |
1-Hexanol, 2-ethyl |
000104-76-7 |
|
1.16 |
3-Octene, |
014919-01-8 |
|
1.17 |
2-Propyl-1-pentanol |
058175-57-8 |
|
1.18 |
1-Hexanol, 2-ethyl |
000104-76-7 |
|
1.19 |
Dodecane |
000112-40-3 |
|
1.20 |
Tetradecane |
000629-59-4 |
|
1.21 |
Benzenamine, 3,5 |
000626-43-7 |
|
1.22 |
Silane, dichloro(3-chloropropyo) |
003401-26-1 |
|
1.23 |
1H-Iden-1-One, 6 (dimethylamino) |
058161-22-1 |
|
1.24 |
3,5-Dichlorophenyl ethylamine |
1000306-63-2 |
|
2.0 |
Parts Per Millionth |
|
|
2.1 |
2-Pentanol, propanoate |
054004-43-2 |
|
2.2 |
2-Bromo dodecane |
013187-99-0 |
|
2.3 |
Octadecane, 1-(ethenyloxy) |
000930-02-9 |
|
2.4 |
2-Methylpentyl isovalerate |
1000236-38-7 |
|
2.5 |
Eicosane |
000112-95-8 |
|
2.6 |
N1-(m Tolyl)-N2-tetrahydro |
332065-24-4 |
|
2.7 |
Azetidine, 1-me |
004923-79-9 |
|
2.8 |
Cyclobutane, methylene |
000292-64-8 |
|
2.9 |
Benzene, 1,1-(1,3butadiyne-1,4) |
000886-66-8 |
|
2.10 |
Tricyclodecanedimethanamine |
026655-37-8 |
|
2.11 |
6-Octadecenoic acid |
000593-39-5 |
|
2.12 |
Cyclohexane, 1-pentyl |
015232-85-6 |
|
2.13 |
4-Pentenal, 2-methylene |
017854-46-5 |
|
2.14 |
3-Hexadecene |
034303-81-6 |
|
2.15 |
2,5-Furandione, 3-(dodecenyl) |
025377-73-5 |
|
2.16 |
Cyclohexanone,4-acetyl |
005034-21-9 |
|
2.17 |
Oxalic acid, 2-ethylhexl isohex |
1000309-38-8 |
|
2.18 |
Benzofuran-2-one, 4 amino-2 |
1000129-51-7 |
|
2.19 |
1,5,9-Cyclododecatrine |
000676-22-2 |
|
2.20 |
Carbonic acid, decyl phenyl este |
1000314-57-4 |
|
2.21 |
Dicyloxybenzene |
035021-67-1 |
|
2.22 |
Carbonic acid, hexadecyl phenyl |
1000314-58-0 |
|
2.23 |
Carbonic acid, Octadecyl pheny |
1000314-58-1 |
|
2.24 |
Carbonic acid, phenyl tetradecyl |
1000314-57-8 |
|
2.25 |
Phynl 3-deoxy-alpha,-d-ribo-he |
1000133-06-1 |
|
2.26 |
6,7-Dioxabicylo(3.2.2)non-8-ene |
006786-21-6 |
|
2.27 |
Beta-d-Mannofuranoside, phenyl |
093524-17-5 |
|
2.28 |
N-Vinyllimidazole |
001072-63-5 |
|
2.29 |
Decanedioic acid, bis (2ethylhex |
000122-62-3 |
|
2.30 |
Glutaric acid, mono-phenyl ester |
037526-03-7 |
|
2.31 |
Carbonic acid, Phynl undec-10-e |
1000314-57-5 |
|
2.32 |
Heptadecane, 2,6,10,14-tetramethyl |
018344-37-1 |
|
2.33 |
Oxalic acid, dodecyl 2-ethylhexy |
1000309-39-5 |
|
2.34 |
5-Chlorobenzo(1,2,5)thiadiazol-4 |
1000311-77-9 |
|
2.35 |
Hexadecane, 1-chloro |
004860-03-1 |
|
2.36 |
6-Nitroundec-5-ene |
1000192-40-3 |
|
2.37 |
Butanedioic acid,2,3,dihydroxy |
013811-71-7 |
|
2.38 |
Isoquinoline, 1,2,3,4-tetrahydro |
029726-60-1 |
|
2.39 |
Pyrrolidine, 1-methyl-3-2-spiro |
04029-14-1 |
|
2.40 |
Imidazo(1,2-b)1,2,4, triazine,6 |
094103-49-8 |
|
2.41 |
2 Propyn-1-one, 1-(2-thienyl) |
056588-20-6 |
|
2.42 |
E-11-Hexadecen-1-o1 |
1000130-89-8 |
|
2.43 |
9-Octadecoi acid |
000112-79-8 |
|
2.44 |
Octadecane, 1-chloro |
003386-33-2 |













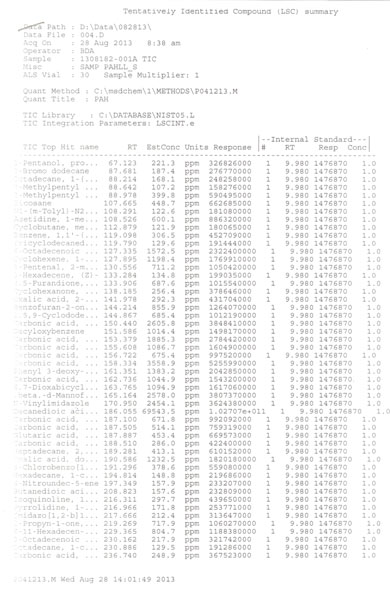
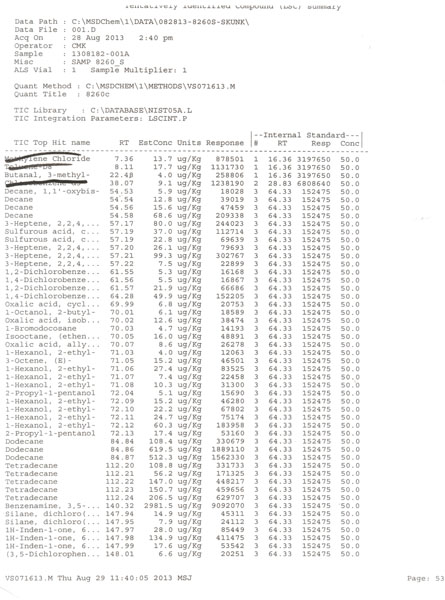


Anyone following this tech is making dirty extract full of residual solvents. Get your stuff tested people! Thats why this is becoming illegal in so many states. These guys are not doctors or chemist or pharmacist. There tools of the gas company and industrial tool manufacturers Bio tech means nothing more than a few months weeks or hours of vocational training Listen to your doctors people. Find a safer extract method there are dozens This is a huge public saftey and health concern Bho will cause serious problems mental, psychological, cardiovascular, resperatory, CANCER! The list goes on. Read clinical studies. They dont turn up in your google searches.
What are your credentials and specifically which clinical studies are you referring to? We aren't doctors, but have certainly involved a number, including PhD's in chemistry on this project, and surprisingly our recent biotechnology graduate did have to pass his organic and biochemistry classes and lab to get the degree. Though not a partner, we have been both supported and helped immensely by the involvement of Dr Kate, Pharm D, whom you note has postings on our site. Without having a clue who we are, you label us with the epithet, "tools of the gas company and industrial tool manufacturer", which not only demonstrates your ignorance, but your lack of integrity. More specifically that you will say anything to make your case, even if you haven't researched it yourself to insure you know what you are talking about. For instance, aside from us having no connections with the gas or industrial tool manufacturers, I opine the reason it is becoming illegal to extract BHO in an unlicensed facilities, is more related to explosions in bathrooms, garages, and motel rooms. Possibly a surprise to you, but my guess is those BHOtard's aren't having their product tested before selling on the black market. Are you aware that here in Oregon, that 100% of the concentrates sold to the public through dispensaries is tested and certified by a third party lab for content, residual solvent, mold, and pesticides and most of it is extracted using BHO techniques? I see the smoke brother, but no smoking gun, only you with an array of mirrors in your hand. GW
Wow, what a write up... tons of information to ponder, thanks again Skunk Pharm...
Wouldnt sterile syringe filters filter out all the petroleum things? I mean the mystery oil is simple bolt grease they probably use somewhen producing the metal of the cans.
You can't mechanically remove other oils from the extract.
Mechanical methods will not work to separate oils that are miscible.
[…] After removal from the crude oil, the gases are typically de-sulfurized using steam and a catalytic reactive bed, and fractionally distilled into the four basic gases. As fractional distilling separates the gasses by specific gravity, the principal contaminants in n-Butane at that point, will be Iso-Butane, a branched molecule isomer of n-Butane, as well as n-Propane, and Cyclo-Propane, plus low levels of heavier, longer chain molecules than C-4, and which are often referred to as Mystery Oil. […]
I like how the comments section starts out with the typical trolling and bullshit, and ends with a number of people begging for their SOC to be tested. Great work, and cheers. Does anyone know the gas ratios of Lucienne? It is a blend of Iso, N, and Propane, but the ratios are kept as "trade secret."
@ Skunk Pharm Research,LLC So every brand of gas is harmfull to make BHO?or just the amount you vape BHO?
All canned gas has residue
As long as you use quality butane, it's not over blasted with lots of extra butane, and properly purged you won't have any negative health affects
And mystery oil isn't much of a mystery, it's the hydraulic and pneumatic oil from the machines that make, package, and pressurize the cans.
I've been doing BHO runs for about 11 years and none of this was available at the beginning. I can say thru my own experimentation that your results are pretty accurate. I have found Lucienne to be the best quality, readily available butane. Although, not as readily available, capital butane has always had the purest results in the end product. I wouldn't use anything other than these two for my own extracts, but I have used other brands for other people, but only after thorough explanation of why I only use Lucienne or capital myself. Newport and power seem to be pretty good, but i still wouldn't use them for myself, especially since I used Ronson on my first couple runs unfortunately.
First time using Colibri to fill a closed loop system... I have never seen SO much mystery oil! Ran and cleaned the system 3 times so far and every time have soaked a 6x6" paper towel with the residual mystery oil left in the collection vessel. I was not sure about what it was till I re-found this post. Will be giving it a couple more dry runs this eve after it cools down to see how much comes out before running any material. I though that the mystery oil was removed after the first run in a closed loop system. Any thoughts on why I have continued to collect the same amount from 3 runs? ~Back to Whip It and Ultra Pure i'm thinking... maybe try that Lucienne, but never the Colibri again. Thank you again Skunk Pharm! ~Dank Granny
Forgot to mention that 36 - 300ml cans were used in the filling of this system.
mystery oil, well its no mystery folks it full of markers put in by the feds, all extracts using it will turn into a lead for lawdogs to come after you for selling it. the gas may be triple distilled, but the mystery oil is then added after that, or if you trust your own brain, how could it be triple distilled and have the mystery oil still in it?answer it wouldn't unless some one added it after the fact. wake up its bad news from the bad idea factory ,the government. all else is BS, only way for it to be there is added after. a bunch of nice theories here, but no thinking...wake up
I will repeat for the 1000 time, all of the cans are bad news, all the rap on them about triple distillation being done is leaving out the info that the harmful things go in afterwards. NO CAN is good, period as in NONE>I have tried for years now to bring awareness to this.
Not making bho anymore orJust smoke the weed then?
Just eat it.
Wow... am to conclude from your chart that plain old Zippo is cleaner, as far as residue, than Puretane? I feel like I bought my butane from McDonalds... like I fell for good advertising. I will study a bit more.
If you do end up getting open blasted BHO, is decarboxolating it going to remove this nasty mystery oil? Or should i just throw it away? The end product would be a topical rub on oil. Would this be bad to use on my skin?
It depends on how much you have in your concentrate. What are you using to open blast with? Decarboxylating per se doesn't, but the heat would evaporate away some.
Cook it into some coconut oil.
I landed on this site as the result of some really pretty, golden looking crumble that when burned smells like rubber tire fire. Not pretty. Tastes like it too. Any idea where this is coming from? This is the 2nd time in 2 weeks that I've been provided with this bunk stuff. We will not be consuming any of it -- at least vaped. Probably going to use it for cooking or something.
By the way, thank you for your work and contributions!
Dirty pump got lubricant into the product creates burnt tire taste
You're welcome!
that taste/smell is from the pump the extractor used being dirty and getting lubricant into the product.
None distilled PHO from ecogreenindustry. Plants that grower burned sulfer in the room during flower? I would ask the bud tender if they have a clue. I would also probably return it to the vendor.
What did you use for butane and did you predistill it before using it to extract?
[…] eine Verunreinigung im Promille Bereich in seinem BHO. Das ganze ist empirisch nachgewiesen. BHO Mystery Oil | Skunk Pharm Research LLC Unter dem Begriff "mystery oil" findet ihr noch mehr dazu. Vakuum oder Ultraschall sind […]
Thank you so much for your advice I was about to order some. I will probably pass for now
I would like to hear your opinion about the MZ12X organic solvent www.purezho.com thank you for everything you have done here its very helpful and appreciated.
The MZ12X that we subjected to a third party forensics lab for analysis, showed it to be Dimethyl Ether and CO2. Dimethyl Ether can be produced any number of different ways, but the MZ12X is manufactured from Methane harvested from Korean municipal sewage. http://www.ohio.edu/people/lees1/DME.html It has a boiling point lower than n-Butane at -12.7C/-28.8F), and is relatively easy to purge. The MSDS shows it to be relatively non toxic, at 1000 ppm Workplace Environmental Exposure Levels (WEEL). http://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en&productNumber=295299&brand=ALDRICH&PageToGoToURL=http%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Faldrich%2F295299%3Flang%3Den It isn't friendly to elastomer seals and gaskets, which makes reclaim difficult and you have to be careful what you store it in. His ad playing on heavy metals from butane is BS. n-Butane is a simple fully saturated alkane, which is harvested as a gas and compressed into a liquid, so is an unlikely source for heavy metals, nor have any of the third party forensic lab shown them to be present. I wouldn't expect heavy metals from the municipal sewage to find their way into the Dimethyl Ether either, nor did we find any. Heavy metals typically come from plant food and fertilizers. He says it is less flammable than butane, but both butane and dimethyl ether are rated under H220 as Class 4 Extremely flammable gases. https://www.airgas.com/msds/001007.pdf http://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en&productNumber=295299&brand=ALDRICH&PageToGoToURL=http%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Faldrich%2F295299%3Flang%3Den Its cousin Diethyl Ether forms explosive peroxides, but no such caveat exists for Dimethyl Ether. Its cousin Diethyl Ether is on the federal watch list, but not Dimethyl. Until we can reclaim it, we will continue to use n-Butane and Propane. Open extraction is not as safe and is more polluting to the environment.
Methane from Korea municipal sewage ? You are spreading misinformation for a public company. Would you like to wage on that Gray wolf?
I've scanned the BHO Mystery Oil article several times for such a comment, and conclude you must be lost. Might you be more specific at the appropriate place? GW
Dimethyl Ether and CO2 are relatively non toxic, but still highly flammable. Literature suggests that it doesn't as readily form peroxides as Diethyl Ether and not on the DEA watch list like Diethyl Ether. It is also hard on elastomer seals and gaskets, so recycling is more difficult. That, and the price makes it relatively expensive to extract with.
your knowledge will pave the way for future generations to come. really inspired by this website.
Also want to say thank you so much for doing this research when I started making bho 2 years ago their was no research available and little speculation oh how to go about the process.
Thanks for the good thoughts Zach!
You state pulling a vacuum will remove the inert chemicals could you do a study on weight of product compared to time needed to remove said chemicals Under vacuum
BULLSHIT!
Someone said if you open blast and put the BHO into an oven at 250* for 15 min it will purge off 100% of the butane and decarb. the oil at the same time. Would the butane and the mystery oil boil off totally at those temps?
Explosion waiting to happen! Never purge butane in an oven!
Im saying after its totally purged. Sorry i wasnt clear on that. We would First purge the BHO in a vac oven for 24 hours then proceed to decarb it in a oven at 250* for 15 min. My question is. Will the mystery oil and other toxins boil off at 250*
No, some of the MO constituents have a boiling pint higher than 250F. Better to take then out before extracting with the butane.
At 50°C (122°F) THC-Acid decarboxylates as the water molecule held in the carbonate form evaporates. This activates the THC. At 31°C (87°F) the less volatile terpenoids start to evaporate, lending the air even more pungent odors.
if people use no "wulfsche Flasche", thy can got mystery`s too ... http://www.reiss-laborbedarf.de/uploaded/image/shop/laborkatalog/654/00085_031_1_small.jpg
Looks like a nice vacuum trap.
Yes it is ;) so in EU it is not allowed to use vacuum pomp's for medical or food production with oil inside, if there is no gas-washer in line... ! and dry-pomp`s are expensive ...
At this point we should be calling it "mystery solved oil" or something to that effect. After all of the work from Skunk Pharms and SkyHighler from IcMag there really sin;t much of a mystery left any more. You guys deserve a lot of thanks and credit for actually doing a bunch of lab tests and flanging up real evidence and publishing it all online.
Thanks for noticing! Some look for solutions, and some look for the imperfections in solutions. Motives generally determine the difference.
[…] http://skunkpharmresearch.com/bho-mystery-oil/ […]
[…] residuals". Hier wurden die "resuduals" extrahiert und labortechnisch untersucht. BHO Mystery Oil | Skunk Pharm Research LLC In summary, there were simple Alkanes present as long as C-16, which are not of health concern […]
because not every brand of every can has the same exact amount of "mystery oil" in it. Mystery oil is not regulated by how much weight per can, its just in there.
After reading the additional work by Skyhighler I am a tad confused as to how the Ronson came out as one of your worst and for him some of the best? That's a pretty substantial difference. Since it is US sourced are there separate locations where they're produced?
because not every brand of every can has the same exact amount of “mystery oil” in it. Mystery oil is not regulated by how much weight per can, its just in there.
Ronson brand was purchased and is no longer the same product.
did you think it is enough, to distill the gas onetime before using it, to get out all non gas shit?
You can distill it down into the parts per billionth level, which is below any levels of official health concern.
any testing on honey 7x
i am using Blue 9x any results on that one?
can you please review Cloud Fuel its supposedly pharmaceutical grade or at least it claims it is on the can, also please do one on DODO please wanting to know the residuals on it begin that its the cheapest around would like to compare it to colibri
Have you guys tested MZ-12x yet? And if so, could you post results? I just found it online and am wondering about it.
see http://skunkpharmresearch.com/2014/02/14/2307/
I just got ripped off by these guys. The website does not function after you register. They do paypal and then once you pay, you never get a response. Thomas McCully is the name you get for payment. Email is Tom.Cimino@gmail.com. The other possibly ficticious names are Nate Diaz and mmoratings@gmail.com. Total ripoff.
Please remove the previous post. They finally contacted me with a tracking no. Lets hope it was what I ordered. Literally a minute after I posted this, they called back. Finally.
We were unable to copyright our name, because we are cannabis related and it is against federal laws. This is obviously not us, but some unscrupulous opportunists.