Cannabis produces phyto cannabinoids in a carboxylic acid form that are not orally active at least at the CB-1 receptor sites, because they don't readily pass the blood brain barrier in their polar form.
To enable them to pass the blood brain barrier, they must first be decarboxylated, to remove the COOH carboxyl group of atoms, which exits in the form of H20 and CO2.
Decarboxylation occurs naturally with time and temperature, as a function of drying, but we can shorten the amount of time required considerably, by adding more heat. The more heat, the faster it occurs, within reasonable ranges, and in fact occurs spontaneously when the material is burned or vaporized.
There is another mechanism at play however, which suggests that we need to control the decarboxylation temperatures carefully.
When we heat cannabis to convert the THCA and CBDA into THC and CBD, we are also converting THC to CBN at a faster rate. At about 70% decarboxylation, we actually start converting THC to CBN at a faster rate than we are converting THCA to THC, so as you can see by the following graph, after about 70% decarboxylation, the levels of THC actually start to fall sharply.
That of course means that the CBN also begins to rise and the medication is becoming more sedative.
Thank you Jump 117 for this excellent graph!
Another fly in the ointment, is that we can never know for sure exactly what the starting state of decarboxylation is, so the times at temperature shown on the graphs are an average.
We can't expect dry material placed in an oven at any given temperature to be that uniform temperature throughout instantly upon placing it in a heated oven, nor know for sure the state of decarboxylation by simple observation.
Decarboxylating plant material, also alters the taste (roasted/toasted), which some find less agreeable, and of course decarboxylating also evaporates away the smaller Monoterpenes and Sequiterpenes alcohols, phenols, ketones, aldehydes, ethers, and esters.
The good news is that it is dirt simple to monitor the state of cannabis oil decarboxylation placed in a 121C/250F hot oil bath, because you can watch the CO2 bubble production.
Just like the curves suggest, CO2 bubble production will proceed at its own observable rate. By keeping the puddle of oil lightly stirred on the bottom and in the corners of the pot (I use a bamboo skewer), so as to keep the bubbles broken free and floating to the top, you can tell exactly when the bubble formation suddenly tapers off at the top of the curve.
That is the point that we take it out of the oil for maximum head effect, and we leave it in until all bubbling stops, if we want a more sedative night time med.
Here are a couple pictures of what oil looks like when boiling off the residual butane. Residual butane or alcohol produces larger, randomly sized bubbles, and is fully purged, when they cease.
I am seemingly missing the middle picture of the CO2 bubbles, so I will add it later, but the second picture shows what fully decarboxylated oil looks like.



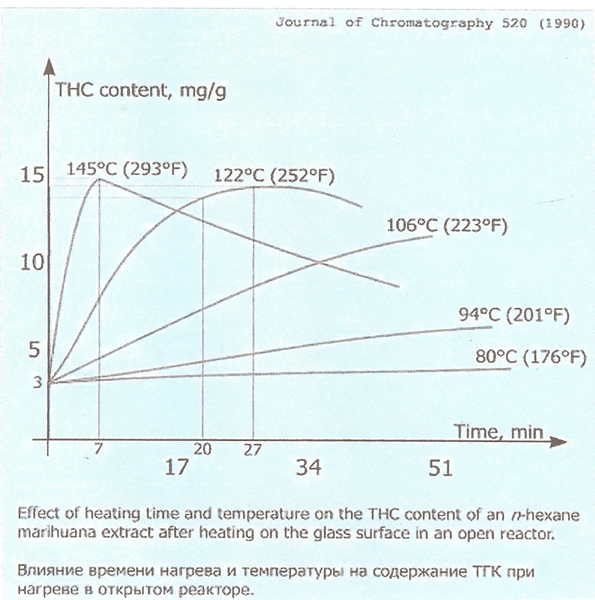





It also starts at 3, and goes up yo 15. So maybe 5x more is the ratio?
The decarb graph is scaled wrong. It shows the peak concentration of THC in the oil is 15 mg per gram. I would expect a multiple of maybe 50, i.e. a yield of 750 mg per gram. If the scale is wrong, how valid are the curves? What can be trusted in this graph?
Don’t air purge. It’s bad for our ozone layer and dangerous. Invest in or build a closed loop extractor. Or better yet, let the big boys do all the hard work and buy your concentrate at a dispensary. You’ll still save big over what they charge for vape cartridges.
The problem is that the famous decarb time/temp graph is based on a hexane extract on a hot plate and not bud/leaf. It takes more time for the heat to penetrate the more intact trichomes that are somewhat more insulated by plant cellulose and air. Also, oil doesn’t extract cannabinoids as effectively as solvents, so you might want to try a doing a double extraction using about half the quantity of fresh oil on the second run. I’d give it another 30 then combine the oil batches.
Whatsapp: +195.464.18238 Open link https://sites.google.com/view/nembutal-pentobarbital-sodiums Buy Xanax bars , Lean syrup , Oxycodone , Adderall , Percocet , Methadone , Ecstasy , Lsd and acid , Actavis Promethazine Cough Syrup , Viagra , Vyvanse , Klonopin , Fentanyl , Rohypnol (Roofies) , Ketamine , Hydrocodone 10/325, MDMA (molly) crystal and pill form , Crack Cocaine , Heroin (white, brown and tar) Weed , Marijuana , Cannabis buy in Switzerland , Germany , Spain , Italy , Strawberry , Sour Diesel , Jack Herer , Durban Poison , Haze, Pineapple Express , Blue Dream , Purple Haze , AK-47 , Grapefruit , White widow , OG Kush , Purple Kush , Black weed , CBD Oil , Hemp oil , Moonrock for sale in Switzerland , Germany , Spain , Italy ,E-mail: danny@ doctor. com https://onlinecannamedshop.com
I recently tried using a pressure cooker to do an oil extraction. I added about 10 grams ab ground cannabis buds to a cup of coconut oil and placed inside the pressure cooker and then cooked it for 30 minutes. I did not tightly seal the jar in order to prevent the lass from cracking due to temperature differences inside and outside the jar. The temperature is supposed to be about 240-250 degree Fahrenheit. The oil did not seem to be very potent, but I am not sure of the THC/CBD of the cannabis, which was homegrown. I am guessing about 10%. Do you see any problem with this procedure? The advantage of this is that one can control the temperature and time and not have to monitor with a thermometer.
I am making concentrate for use in a low wattage vape pen, using the disposable batteries because the higher wattage batteries burn up the atomizers in small carts so with this being said I will need to decarb right? Probably making my concentrate using grain alcohol wash and was going to let it air purge or cook it off ( haven't decided yet) is there an advantage to either other than the obvious fact that cooking it off gets done quicker. Any advice would be greatly appreciated. This will be my first run with this so any help on some of the best methods that others have already come up with through trial and error would be great. I have done hours and hours of research but there is some conflicting stuff out there.
Finally a vendor that actually tests their product. Cant recomend these guys enough. Rsoforall.webnode.com
I have thc a coconut oil....I did not decarb....can I leave in crockpot and heat up more to decarb now?
I just recently have came across 2g of bho. I have come to the decision that it hasn't been purged properly or hasn't been purged enough and still contains butane. Will decarbing this bho make it safe to consume or should I chalk this up as a lose and move on?
I am trying to make a potent extract for e-juice and was wondering. Is it necessary for me to decarb the extract even though it will naturally decarb when the heat from the atomizer heats it up giving me thc burning off the thc-a?? Or did I miss something I made some but did not get the desired results. The first time around I had my plant materials extracted at 180f-190f for roughly 45 minutes.
I have some readers asking about a variation of this. If you put bud in coconut oil and heat it, can you tell what stage of decarb it is in by the bubbles being released? If so, shouldn't we skip oven decarb completely and decarb straight in oil?
[…] of the cannabinoid family. In order for CBC to even exist in cannabis, the cannabis must go through decarboxylation. This particular cannabinoid shows great promise in treating cancer; it is said to inhibit the […]
Hello are you looking for quality and pure cannabis oil email Dr Richard @ drrichardmedicaloil@gmail.com i can assure you that, this is where you can get your pure hemp from.
Thanks for the great site. I'm wondering if you have a citation for the claim that THCA cannot cross the blood-brain barrier? Thanks again... Hi Im not the Poster of the aBOVE question. I just surfed randomly your site and could EDIT the comment from other user. I have a question too. Can you kindly explain how to properly decarb ice-o-lator (ice hash) - I want to make an 100% alcool extraction out of it. Also does ultrasonic speed up the process ? Thank you best Stefan
Not off the top of my head but I'll run it by our pharma brain trust and see what resources they might have to offer. GW
Thanks. I was having dinner with a friend the other night (a PhD Nat. Prod. Chemist/cannabis expert) and we were talking extracts and THCA vs THC and I repeated this idea that THCA needs to be decarbed to pass the blood brain barrier. I'd read it here and on Evio Labs' website. I had it planted in my mind I'd also heard Ethan Russo repeat this. Anyway, my friend asked me if I was sure about this fact. I said I'd verify it. Well, I've searched much of the research I have access to and, for the life of me, I cannot support it with a single research paper. If I do find something authoritative to support it, I'll get back to you. If you could do the same, I'd appreciate it.
My only research is that when I heat my cannabaniods I get high! Lol but
Ok, I just found it. I did hear it first from Ethan Russo. It's the Moreno-Sanz THCA-A paper from last year (ref. below). According to Moreno-Sanz, THCA-A doesn't cross the blood-brain barrier, hence providing peripheral nervous system effects, without the CNS effects. Dr. Russo suggests something associated with epilepsy's etiology may allow it to cross, which is an interesting suggestion. Moreno-Sanz G. 2016. Can You Pass the Acid Test? Critical Review and Novel Therapeutic Perspectives of Δ 9-Tetrahydrocannabinolic Acid A. Cannabis and Cannabinoid Research 1:124–130. [ PDF: http://bit.ly/2pM8igE ]
This is good news, I'm a firm believer in full plant extraction. I include decarb, and undecarbed cannabis in my extractions. We have been using Raw Cannabis for years. Everyone should be consuming Cannabis raw everyday. Zack.
Wondering if anyone making tinctures with buds has any experience with decarbing half the bud and leaving the other half natural. The desired effect being to utilize the cbda and thca along with the thc and cbd. I've read they all have their own medicinal properties. Thanks
Check out Royal Jelly, a mixture of THC Delta 8 or 9 Clear distillate, mixed with THC-a crystals. https://skunkpharmresearch.com/royal-jelly/ We've had patients dose with HS Oil and juice at the same time, which would also achieve the same effects. Check out Pharmer Kate's article on the medicinal properties of the carboxylic acid forms of THC and CBD. https://skunkpharmresearch.com/cannabinoid-carboxylic-acids-thca-and-cbda-their-potential-functions-applications-and-methods-of-extraction/ GW
A great technique for a whole plant medicine, I've done this with coconut oil. 3/4 of total weight decarb. 1/4 use raw or non - decarb cannabis. If using raw or fresh cannabis with coconut oil, I would suggest refrigerating your end product in case of water residuals. Z. Membrino
Please note: using solvents such as butane or isopropyl alcohol is not safe for human consumption NO MATTER WHAT!!! Only food grade solvents are safe especially when making concentrates, Simpson wrote the Bible but he advocates the use of harmful solvents to extract the oil. The cleanest oil is made with 190 proof everclear. If you are making meds for one one with FOOD FOR THOUGHT FOLKS!
Butane is rated GRAS or Generally Regarded As Safe by the FDA, who also rates Isopropyl as a Class III solvent. Solvents in Class 3 (Table 3) may be regarded as less toxic and of lower risk to human health. Class 3 includes no solvent known as a human health hazard at levels normally accepted in pharmaceuticals. However, there are no long-term toxicity or carcinogenicity studies for many of the solvents in Class 3. Available data indicate that they are less toxic in acute or short-term studies and negative in genotoxicity studies. It is considered that amounts of these residual solvents of 50 mg per day or less (corresponding to 5,000 ppm or 0.5 percent under Option 1) would be acceptable without justification. Higher amounts may also be acceptable provided they are realistic in relation to manufacturing capability and good manufacturing practice (GMP).
When you say soak in oil bath at 250 do you mean the cannabis oil has to get to 250 as well then start the 20 min clock? Reason I asked is I did this after the hot extraction method (at 180f) and did not see those big bubbled in your picture a very few small ones. After about 30 mins in oil bath I stopped.
Good Stuff, thanks!! Any idea if decarbing this way vs the oven method affects the taste/color for vaping? Wasn't sure if this way make it taste worse and darker or taste better while you said preserving more terpenes.
Am I able to use live resin or live rosin? Should I decarboxylate it or since it's live do I not have to? If I do have to cook it what should the temperature be and should I use a double broiler to cook it or can i just put it in the over as is? Thanks!
The THC/CBD in Live Resin and Rosin, are in carboxylic acid form (THC-a/CBD-a). If your intended use requires decarboxylation, than yes you will need to do so. GW
Hello. I tried to decarb 220mg of bho to whip up some jolly ranchers. I was using a recipe and it had me decarb my bho in a toaster oven at 200F for 20 minutes. I lost a lot of bho in that process. Can anyone tell me the issue there? It's really confusing seeing that happen but then reading from other sources to heat it even higher and longer.
Toaster ovens temp is very uneven usually. Get yourself an oven thermometer (like $5) to make sure it is really the temp you want. Most likely, unless the temp was much higher than you thought, you lost solvents or water that was in your BHO.
There are some losses with decarboxylation, in that you are removing one carbon atom, two oxygen atoms, and a hydrogen atom from each C-22 molecule when you decarboxylate. It goes from a C22-H31-O4 molecule to a C21-H30-O2 size/weight molecule. 200F for 20 minutes is not optimum for highest THC levels. Is there a reason you chose that particular curve? As noted toaster ovens have little control or uniformity at those temperatures. How are you controlling/measuring? What was the oil container and what happened when you lost the material? GW
Translation: "Where's the data?"
Which data? GW
PLEASE EXCUSE THE CAPS DUE TO POOR VISION , I WANT TO MAKE GUMMY CANDY REGULAR OR SOUR.AND PREFER NOT TO USE BHO JUST 99% iso, SO AFTER WASHING/ RINSEING AS EXPLAINED BY SKUNK GW.IS 12 HRS ENOUGH TIME IN FREEZER AND DO I PUT MY 3 JARS ON TOP OF THR SOLID BLOCK OF ICE DURING THIS TIME.? THEN TAKE 3 PYREX PANS AND WATCH FOR 127- 130 DEGREES AS THE PURGING BEGINS TILL THE BUBBLES STOP? LET COOL TILL CRISPY, OR WORKABLE TO THE TOUCH? THEN HOW MUCH IN WEIGHT DO I ADD TO THE RECIPE ,WHICH CALLS FOR 4 STRAWBERRY JELLO BOXES AND ONE BOX OF GELATIN.?AS I WANT VERY POTENT CANDY FOR SLEEPING. MEANING 1 FULL PIECE FOR SLEEPING, AND HALF FOR A GOOD OLE TIME? UNLESS YOU HAVE A BETTER RECIPE FOR ME TO USE ,I AM SKILLED IN THE KITCHEN IN ALL ASPECTS EXCEPT MAKING CANDY BUT CAN FOLLOW DIRECTIONS VERY WELL, HAVE MADE MANY BAKED GOODS THAT SOME FRIENDS NORMALLY WOULD NEVER EAT TILL I PROMISED THEM THESE ARE NOT THE BAKED GOODS YOU HAVE HAD IN THE PAST. AND THEY ARE A HIT BUT I FEEL I AM MORE THEN READY FOR GUMMY CANDY IN ANY SHAPE BUT NEED A GUIDE FOR THE POWER BEARS, SHOOTING STARS,YOU GET IT. NOT FOR BEGINERS. LOL PLEASE HELP ME WITH THIS THE HARDEST PART IS EITHER USING TINTURE OR THE OTHER? SHATTER WAX.YOUR ADVICE HAS ALWAYS BEEN GREAT AND WIYH A QUICK REPLY. AS ALWAYS, THANK YOU FOR YOUR TIME, AND HOPE YOU CAN ADVISE NO DOUBT. NEWEYGOOEY. ..PLEASE DO NOT SHARE MY EMAIL AS I WAS THE VICYIM OF A VIOLENT CRIME,NEED PRIVACY.HOPE THIS DOES NOT BOTHER YOU, THANK'S
SORRY, NONE OF US HAS MADE GUMMY BEARS, BUT CAN TALK IN GENERALITIES, ABOUT DOSAGE. WE'VE FOUND ABOUT 100 MG OF AVERAGE CONCENTRATE TO BE AN AVERAGE DOSE, BUT SOME BROTHERS AND SISTERS HAVE ULTRA LOW TOLERANCE, SO SUGGEST THAT YOU START AT ABOUT 25% OF THAT AND TITRATE UP TO YOUR DESIRED DOSAGE. IN SOME LOCALS LIKE WASHINGTON, YOU ARE ALLOWED 10MG THC PER SERVING, AND UP TO 10 SERVINGS PER PACKAGE. ICMAG IS ONE OF THE LARGER ONES AND HAS THIS THREAD ON GUMMYBEARS: https://www.icmag.com/ic/showthread.php?t=294359 GW
so to decarb BHO, I need to add in the coconut oil to the BHO before , even when using it to make coconut oil? and if so how much coconut per gram for decarb?
Easier to decarb the oil all by itself, by placing a container of into a 250F hot oil bath. GW
Would I be able to decarb bho without a solvent? And what would be a good ratio/process for using vegetable glycerin or propylene glycol as a solvent for decarbing?
No solvent is required for decarb. Just stick the container of oil in the 250F hot oil bath and it will liquify all by itself. GW
Thanks so much! Just a quick question, where would be a good place to find the proper method for a hot oil bath, and proper ratio's and process for using glycerin or glycol as a solvent?
Coconut oil has a higher fat content, better extraction.
So I should decarb a gram of oil in my oven at 250f for 25 minutes? My recipe calls for butter but I see coconut oil mentioned here quite a bit. What is the reason for this? Should I substitute half coconut oil?
We've had good success with either ghee or coconut oil. Both are tasty and effective. Coconut oil contains medium chain triglycerides. Check out our recipe for ghee extraction, using no water to minimize water soluble and chlorophyll pickup. https://skunkpharmresearch.com/extracting-with-oils-and-fats/ GW
I'm starting from a gram of bho so is there a benefit to the coconut oil since it is already extracted? The 250 degrees for 25 minutes sound right?
The coconut oil helps with absorption and ties up the liver processing it, so that the cannabinoids stick around longer. Possibly closer to 30 minutes. GW
Was wondering how long it takes to Decarb naturally?
Cannabis Decarbs naturally when being cured or dried, This step takes around 3 weeks, for proper drying and curing. But at this point you still need additional heat or time for it to actually decarb for edibles.
Sea Maiden ages her glycerine extractions a year to naturally decarboxylate them. Clearly it would depend on the storage conditions. GW
I'm going to be decarbing ~8g oz total of shatter and budder in the oven, what temperature should I use and how long (not using the oil method you suggested)? Afterwards I'm going to infuse it into coconut oil over a double boiler. I did the math and I have ~6400mg of thc to work with ideally so at 40mg a cookie I'm hoping to get around 160 cookies. How much coconut oil should I use and how long should I let it infuse for? Also how much lecithin should I add?
Send me one of them cookies...lol
Can you decarboxylate a thc-a tincture? If so, how?
Depends on the boiling point of the solvent the THC-a is a solute in. Easy with oil or VG, but not so easy in alcohol because of the low boiling point. Stick a container of it 250F vegetable oil for approximately 30 minutes, but you can gauge it precisely by watching CO2 bubble production, as described in the article, GW
Put it in oven at 330 (whole jar an all) leave it for a hr...half that time is the jars coming up to temp
Do the times change if you heat coconut oil to 250-275 and decarb the BHO oil in the coconut oil
Time at heat should be about the same. GW
If I am going to be making things likes brownies, cookies, and such. Would I need to decarb the bho before if it's going to be in an 325-375 degree fahrenheit oven for 20-30 minutes? I feel that it will decarb as it cooks, and have had varying different effects from everything I've made.
The center of the brownies won't be reaching 375 until the end, so it will help but probably won't do a complete job. On the other hand, we made brownies that work for decades before having decarboxylation explained to us, and they worked, demonstrating that it works at least somewhat, if not perfectly. GW
Thank you for all the info! Also I made made some medicated gummy bears yesterday, and the gummy bears I made with bho have completely melted into a red puddle in a ziplock overnight while the ones without are completely fine. I tried to find something on the internet for a couple hours but haven't come across anything. I made hard candy the same way and had no problem with the wax dissolving into the sugar mixture but these just have little pieces of wax in them and on the outside. Would you have any info on the making of gummies? Would I need to use a tincture to incorporate the bho first? Much appreciated skunkpharm you people rock!
I'm wanting to make a THC-A tincture using BHO. Do I still need to decarb my concentrates before infusing with oil?
Is a THC-a tincture the desired end product? What is your desired end use? A THC-a tincture is ostensibly the THC-a, which is not decarboxylated, mixed with the solvent which doesn't need to be, nor can it be. The THC-a molecule is too large to pass the blood brain barrier so as to reach the CB-1 receptors in the brain, so won't be psychoactive. If you decarboxylate the C-22 THC-a, it will revert to its phenolic diterpenoid C-21 form, which would be THC. It will pass the blood brain barrier and produce psychoactive effects. A THC-a tincture must be carefully stored and used fast, because it is unstable and may (will) decarboxylate with time or under certain conditions, thus producing psychoactive effects and making previous dosage calculations moot. GW
Thank you for your response. Yes, a THC-a tincture is the desired end product and will be administered orally for my dog. I had purchased a THC-a tincture from my local dispensary for him that I gave along with an Everclear extract that has cleared a diagnosed osteosarcoma cancer. I'd like to keep using the combination of the two for continued maintenance. The tincture is stored in the fridge to help prevent or prolong activation.
In the fridge, in an opaque or cobalt blue bottle, is a good way to store it. Blue is reflecting the UV end of the spectrum. GW
My plan is to use a Magic Butter machine to infuse the oil. I was going to run multiple cycles. One at 130° for 2-4hrs to blend then a couple 8hrs cycles with no heat to finalize.
To make a ethanol THC-a tincture, you might consider skipping the BHO step and doing a QWET extraction, which you can reduce to the desired concentration. https://skunkpharmresearch.com/qwet-extraction/ GW
Thank you so so much. Your response and information has been and will be incredibly useful. I appreciate you taking the time to help and for your continued research and education in cannabis.
If you want thc-a you MUST NOT decarboxylate and should use as little heat as possible. Kept refrigerated I have had it remain decarboxylated for over three months. As it decarboxylates gradually either you or your dog would have the opportunity to become used to it gradually.
Thank you so so much. Your response and information has been and will be incredibly useful. I appreciate you taking the time to help and for your continued research and education in cannabis.
Hey I'm decarbing some raw hash oil it's been in the oven for about a hour now and there is no sign of bubbling what you thing is the problem.
What temperature? How old was the material and how was it extracted and processed up to this point? GW
Can I decarboxylate raw hash oil in a conventional oven? if so, what temp an for how long? Also, does decarbing raw oil release dangerous fumes i should be careful of?
You can decarboxylate in a conventional oven using the approximate time show on the chart, and can tell where you're at, by watching the bubbles. The CO2 and H2O bubbles released are non toxic and non flammable. GW
Just wondering why you chose to go with 250 instead of 290? Is it because it's easier to gauge when the CO2 bubbles slow down... due to the length of time (7 vs 27 min) and thus easier not to over do it?
Heat does more than just decarboxylate. I use 250F to reduce monoterpene loss. GW